Fluoxetine induces alkalinization of astroglial cytosol through stimulation of sodium-hydrogen exchanger 1: dissection of intracellular signaling pathways
- PMID: 25784857
- PMCID: PMC4347488
- DOI: 10.3389/fncel.2015.00061
Fluoxetine induces alkalinization of astroglial cytosol through stimulation of sodium-hydrogen exchanger 1: dissection of intracellular signaling pathways
Abstract
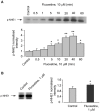
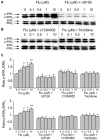
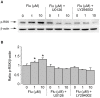
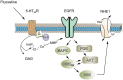
Clinical evidence suggest astrocytic abnormality in major depression (MD) while treatment with anti-psychotic drugs affects astroglial functions. Astroglial cells are involved in pH homeostasis of the brain by transporting protons (through sodium-proton transporter 1, NHE1, glutamate transporters EAAT1/2 and proton-lactate co-transporter MCT1) and bicarbonate (through the sodium-bicarbonate co-transporter NBC or the chloride-bicarbonate exchanger AE). Here we show that chronic treatment with fluoxetine increases astroglial pH i by stimulating NHE1-mediated proton extrusion. At a clinically relevant concentration of 1 μM, fluoxetine significantly increased astroglial pH i from 7.05 to 7.34 after 3 weeks and from 7.18 to 7.58 after 4 weeks of drug treatment. Stimulation of NHE1 is a result of transporter phosphorylation mediated by several intracellular signaling cascades that include MAPK/ERK1/2, PI3K/AKT and ribosomal S6 kinase (RSK). Fluoxetine stimulated phosphorylation of ERK1/2, AKT and RSK in a concentration dependent manner. Positive crosstalk exists between two signal pathways, MAPK/ERK1/2 and PI3K/AKT activated by fluoxetine since ERK1/2 phosphrylation could be abolished by inhibitors of PI3K, LY294002 and AKT, triciribine, and AKT phosphorylation by inhibitor of MAPK, U0126. As a result, RSK phosphorylation was not only inhibited by U0126 but also by inhibitor of LY294002. The NHE1 phoshorylation resulted in stimulation of NHE1 activity as revealed by the NH4Cl-prepulse technique; the increase of NHE1 activity was dependent on fluoxetine concentration, and could be inhibited by both U0126 and LY294002. Our findings suggest that regulation of astrocytic pH i and brain pH may be one of the mechanisms underlying fluoxetine action.
Keywords: AKT; ERK1/2; NHE1; astrocytes; fluoxetine; pHi.
Figures



Similar articles
-
ERK1/2-p90RSK-mediated phosphorylation of Na+/H+ exchanger isoform 1. A role in ischemic neuronal death.J Biol Chem. 2007 Sep 21;282(38):28274-84. doi: 10.1074/jbc.M702373200. Epub 2007 Jul 30. J Biol Chem. 2007. PMID: 17664275
-
Ras triggers acidosis-induced activation of the extracellular-signal-regulated kinase pathway in cardiac myocytes.Biochem J. 2006 Nov 1;399(3):493-501. doi: 10.1042/BJ20051628. Biochem J. 2006. PMID: 16831126 Free PMC article.
-
The Na+/H+ exchanger, NHE1, differentially regulates mitogen-activated protein kinase subfamilies after osmotic shrinkage in Ehrlich Lettre Ascites cells.Cell Physiol Biochem. 2007;20(6):735-50. doi: 10.1159/000110434. Cell Physiol Biochem. 2007. PMID: 17982256
-
Sodium-hydrogen exchange and platelet function.J Thromb Thrombolysis. 1999 Jul;8(1):15-24. doi: 10.1023/a:1008986329267. J Thromb Thrombolysis. 1999. PMID: 10481210 Review.
-
Na (+)/H (+)exchange in the tumour microenvironment: does NHE1 drive breast cancer carcinogenesis?Int J Dev Biol. 2015;59(7-9):367-77. doi: 10.1387/ijdb.140336lf. Int J Dev Biol. 2015. PMID: 26679950 Review.
Cited by
-
Sleep Disturbance in Bipolar Disorder: Neuroglia and Circadian Rhythms.Front Psychiatry. 2019 Jul 18;10:501. doi: 10.3389/fpsyt.2019.00501. eCollection 2019. Front Psychiatry. 2019. PMID: 31379620 Free PMC article. Review.
-
Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases.Biomedicines. 2024 Feb 18;12(2):457. doi: 10.3390/biomedicines12020457. Biomedicines. 2024. PMID: 38398057 Free PMC article. Review.
-
General Pathophysiology of Astroglia.Adv Exp Med Biol. 2019;1175:149-179. doi: 10.1007/978-981-13-9913-8_7. Adv Exp Med Biol. 2019. PMID: 31583588 Free PMC article. Review.
-
Electroacupuncture prevents astrocyte atrophy to alleviate depression.Cell Death Dis. 2023 May 29;14(5):343. doi: 10.1038/s41419-023-05839-4. Cell Death Dis. 2023. PMID: 37248211 Free PMC article.
-
Translational potential of astrocytes in brain disorders.Prog Neurobiol. 2016 Sep;144:188-205. doi: 10.1016/j.pneurobio.2015.09.003. Epub 2015 Sep 16. Prog Neurobiol. 2016. PMID: 26386136 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

