Role of amyloid peptides in vascular dysfunction and platelet dysregulation in Alzheimer's disease
- PMID: 25784858
- PMCID: PMC4347625
- DOI: 10.3389/fncel.2015.00065
Role of amyloid peptides in vascular dysfunction and platelet dysregulation in Alzheimer's disease
Abstract
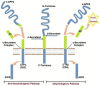
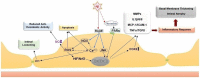
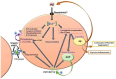
Alzheimer's disease (AD) is the most common neurodegenerative cause of dementia in the elderly. AD is accompanied by the accumulation of amyloid peptides in the brain parenchyma and in the cerebral vessels. The sporadic form of AD accounts for about 95% of all cases. It is characterized by a late onset, typically after the age of 65, with a complex and still poorly understood aetiology. Several observations point towards a central role of cerebrovascular dysfunction in the onset of sporadic AD (SAD). According to the "vascular hypothesis", AD may be initiated by vascular dysfunctions that precede and promote the neurodegenerative process. In accordance to this, AD patients show increased hemorrhagic or ischemic stroke risks. It is now clear that multiple bidirectional connections exist between AD and cerebrovascular disease, and in this new scenario, the effect of amyloid peptides on vascular cells and blood platelets appear to be central to AD. In this review, we analyze the effect of amyloid peptides on vascular function and platelet activation and its contribution to the cerebrovascular pathology associated with AD and the progression of this disease.
Keywords: Alzheimer’s disease; amyloid peptides; cerebrovascular disease; platelets; vascular cells.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

