Terminalia chebula Fructus Inhibits Migration and Proliferation of Vascular Smooth Muscle Cells and Production of Inflammatory Mediators in RAW 264.7
- PMID: 25784946
- PMCID: PMC4345257
- DOI: 10.1155/2015/502182
Terminalia chebula Fructus Inhibits Migration and Proliferation of Vascular Smooth Muscle Cells and Production of Inflammatory Mediators in RAW 264.7
Abstract
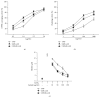
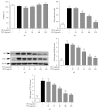
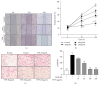
Pathogenesis of atherosclerosis and neointima formation after angioplasty involves vascular smooth muscle cells (VSMCs) migration and proliferation followed by inflammatory responses mediated by recruited macrophages in the neointima. Terminalia chebula is widely used traditional medicine in Asia for its beneficial effects against cancer, diabetes, and bacterial infection. The study was designed to determine whether Terminalia chebula fructus water extract (TFW) suppresses VSMC migration and proliferation and inflammatory mediators production in macrophage (RAW 264.7). Our results showed that TFW possessed strong antioxidative effects in 1,1-diphenyl-2-picryl hydrazyl (DPPH) scavenging and lipid peroxidation assays. In addition, TFW reduced nitric oxide (NO) production, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) expression in RAW 264.7 cells. Also, TFW inhibited platelet-derived growth factor (PDGF-BB) induced VSMC migration as determined by wound healing and Boyden chamber assays. The antimigratory effect of TFW was due to its inhibitory effect on metalloproteinase-9 (MMP-9) expression, focal adhesion kinase (FAK) activation, and Rho-family of small GTPases (Cdc42 and RhoA) expression in VSMCs. Furthermore, TFW suppressed PDGF-BB induced VSMC proliferation by downregulation of mitogen activated protein kinases (MAPKs) signaling molecules. These results suggest that TFW could be a beneficial resource in the prevention of atherosclerosis.
Figures


References
-
- Tanizawa S., Ueda M., van der Loos C. M., van der Wal A. C., Becker A. E. Expression of platelet derived growth factor B chain and β receptor in human coronary arteries after percutaneous transluminal coronary angioplasty: an immunohistochemical study. Heart. 1996;75(6):549–556. doi: 10.1136/hrt.75.6.549. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

