Molecular heterogeneity in glioblastoma: potential clinical implications
- PMID: 25785247
- PMCID: PMC4347445
- DOI: 10.3389/fonc.2015.00055
Molecular heterogeneity in glioblastoma: potential clinical implications
Abstract
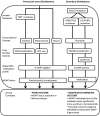
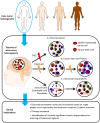
Glioblastomas, (grade 4 astrocytomas), are aggressive primary brain tumors characterized by histopathological heterogeneity. High-resolution sequencing technologies have shown that these tumors also feature significant inter-tumoral molecular heterogeneity. Molecular subtyping of these tumors has revealed several predictive and prognostic biomarkers. However, intra-tumoral heterogeneity may undermine the use of single biopsy analysis for determining tumor genotype and has implications for potential targeted therapies. The clinical relevance and theories of tumoral molecular heterogeneity in glioblastoma are discussed.
Keywords: biomarkers; glioblastoma; glioma; heterogeneity; intra-tumoral heterogeneity; molecular; transcriptional subtype.
Figures
References
-
- Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Nervous System. Lyon: International Agency for Research on Cancer; (2000).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

