Induction of long-term potentiation and long-term depression is cell-type specific in the spinal cord
- PMID: 25785524
- PMCID: PMC4365505
- DOI: 10.1097/01.j.pain.0000460354.09622.ec
Induction of long-term potentiation and long-term depression is cell-type specific in the spinal cord
Abstract
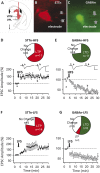
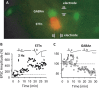
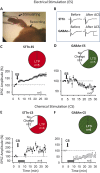
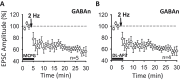
The underlying mechanism of chronic pain is believed to be changes in excitability in spinal dorsal horn (DH) neurons that respond abnormally to peripheral input. Increased excitability in pain transmission neurons, and depression of inhibitory neurons, are widely recognized in the spinal cord of animal models of chronic pain. The possible occurrence of 2 parallel but opposing forms of synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD) was tested in 2 types of identified DH neurons using whole-cell patch-clamp recordings in mouse spinal cord slices. The test stimulus was applied to the sensory fibers to evoke excitatory postsynaptic currents in identified spinothalamic tract neurons (STTn) and GABAergic neurons (GABAn). Afferent conditioning stimulation (ACS) applied to primary afferent fibers with various stimulation parameters induced LTP in STTn but LTD in GABAn, regardless of stimulation parameters. These opposite responses were further confirmed by simultaneous dual patch-clamp recordings of STTn and GABAn from a single spinal cord slice. Both the LTP in STTn and the LTD in GABAn were blocked by an NMDA receptor antagonist, AP5, or an intracellular Ca chelator, BAPTA. Both the pattern and magnitude of intracellular Ca after ACS were almost identical between STTn and GABAn based on live-cell calcium imaging. The results suggest that the intense sensory input induces an NMDA receptor-dependent intracellular Ca increase in both STTn and GABAn, but produces opposing synaptic plasticity. This study shows that there is cell type-specific synaptic plasticity in the spinal DH.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

Similar articles
-
Reactive oxygen species affect spinal cell type-specific synaptic plasticity in a model of neuropathic pain.Pain. 2017 Nov;158(11):2137-2146. doi: 10.1097/j.pain.0000000000001014. Pain. 2017. PMID: 28708760 Free PMC article.
-
Reciprocal Homosynaptic and heterosynaptic long-term plasticity of corticogeniculate projection neurons in layer VI of the mouse visual cortex.J Neurosci. 2013 May 1;33(18):7787-98. doi: 10.1523/JNEUROSCI.5350-12.2013. J Neurosci. 2013. PMID: 23637171 Free PMC article.
-
Non-Hebbian plasticity at C-fiber synapses in rat spinal cord lamina I neurons.Pain. 2013 Aug;154(8):1333-42. doi: 10.1016/j.pain.2013.04.011. Epub 2013 Apr 8. Pain. 2013. PMID: 23707311 Free PMC article.
-
Central neuronal plasticity, low back pain and spinal manipulative therapy.J Manipulative Physiol Ther. 2004 Jun;27(5):314-26. doi: 10.1016/j.jmpt.2004.04.005. J Manipulative Physiol Ther. 2004. PMID: 15195039 Review.
-
Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy.Mol Pain. 2011 Mar 28;7:20. doi: 10.1186/1744-8069-7-20. Mol Pain. 2011. PMID: 21443797 Free PMC article. Review.
Cited by
-
NMDARs mediate peripheral and central sensitization contributing to chronic orofacial pain.Front Cell Neurosci. 2022 Sep 27;16:999509. doi: 10.3389/fncel.2022.999509. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36238833 Free PMC article. Review.
-
D1/D5 Dopamine Receptors and mGluR5 Jointly Enable Non-Hebbian Long-Term Potentiation at Sensory Synapses onto Lamina I Spinoparabrachial Neurons.J Neurosci. 2022 Jan 19;42(3):350-361. doi: 10.1523/JNEUROSCI.1793-21.2021. Epub 2021 Nov 23. J Neurosci. 2022. PMID: 34815314 Free PMC article.
-
Long-Term Depression Induced by Optogenetically Driven Nociceptive Inputs to Trigeminal Nucleus Caudalis or Headache Triggers.J Neurosci. 2018 Aug 22;38(34):7529-7540. doi: 10.1523/JNEUROSCI.3032-17.2018. Epub 2018 Jul 27. J Neurosci. 2018. PMID: 30054391 Free PMC article.
-
Neuron Type-Dependent Synaptic Activity in the Spinal Dorsal Horn of Opioid-Induced Hyperalgesia Mouse Model.Front Synaptic Neurosci. 2021 Nov 18;13:748929. doi: 10.3389/fnsyn.2021.748929. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34867259 Free PMC article.
-
Long-term bilateral change in pain and sensitivity to high-frequency cutaneous electrical stimulation in healthy subjects depends on stimulus modality: a dermatomal examination.Front Med (Lausanne). 2024 Jan 16;10:1337711. doi: 10.3389/fmed.2023.1337711. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38293302 Free PMC article.
References
-
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell 1993;75:1253–62. - PubMed
-
- Antal M, Polgar E, Chalmers J, Minson JB, Llewellyn-Smith I, Heizmann CW, Somogyi P. Different populations of parvalbumin-and calbindin-D28k-immunoreactive neurons contain GABA and accumulate 3H-D-aspartate in the dorsal horn of the rat spinal cord. J Comp Neurol 1991;314:114–24. - PubMed
-
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3',5'-cyclic GMP in cultured hippocampal neurons. Nature 1995;376:74–80. - PubMed
-
- Artola A, Brocher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 1990;347:69–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

