The effects of marine carbohydrates and glycosylated compounds on human health
- PMID: 25785562
- PMCID: PMC4394518
- DOI: 10.3390/ijms16036018
The effects of marine carbohydrates and glycosylated compounds on human health
Abstract
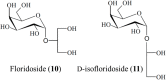
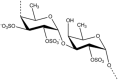
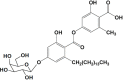
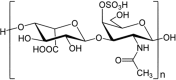
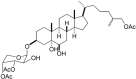
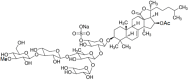
Marine organisms have been recognized as a valuable source of bioactive compounds with industrial and nutraceutical potential. Recently, marine-derived carbohydrates, including polysaccharides and low molecular weight glycosylated oligosaccharides, have attracted much attention because of their numerous health benefits. Moreover, several studies have reported that marine carbohydrates exhibit various biological activities, including antioxidant, anti-infection, anticoagulant, anti-inflammatory, and anti-diabetic effects. The present review discusses the potential industrial applications of bioactive marine carbohydrates for health maintenance and disease prevention. Furthermore, the use of marine carbohydrates in food, cosmetics, agriculture, and environmental protection is discussed.
Figures
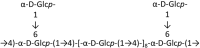







Similar articles
-
Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates.Adv Food Nutr Res. 2014;73:197-220. doi: 10.1016/B978-0-12-800268-1.00010-X. Adv Food Nutr Res. 2014. PMID: 25300548 Review.
-
Biological activities and potential health benefits of bioactive peptides derived from marine organisms.Int J Biol Macromol. 2012 Nov;51(4):378-83. doi: 10.1016/j.ijbiomac.2012.06.001. Epub 2012 Jun 7. Int J Biol Macromol. 2012. PMID: 22683669 Review.
-
Marine peptides and their anti-infective activities.Mar Drugs. 2015 Jan 16;13(1):618-54. doi: 10.3390/md13010618. Mar Drugs. 2015. PMID: 25603351 Free PMC article. Review.
-
Marine Carbohydrate-Based Compounds with Medicinal Properties.Mar Drugs. 2018 Jul 9;16(7):233. doi: 10.3390/md16070233. Mar Drugs. 2018. PMID: 29987239 Free PMC article. Review.
-
Polysaccharides from the Marine Environment with Pharmacological, Cosmeceutical and Nutraceutical Potential.Molecules. 2016 Apr 27;21(5):551. doi: 10.3390/molecules21050551. Molecules. 2016. PMID: 27128892 Free PMC article. Review.
Cited by
-
Protective Effects of Topical Administration of Laminarin in Oxazolone-Induced Atopic Dermatitis-like Skin Lesions.Mar Drugs. 2022 Oct 26;20(11):669. doi: 10.3390/md20110669. Mar Drugs. 2022. PMID: 36354992 Free PMC article.
-
Marine Cyanobacteria and Microalgae Metabolites-A Rich Source of Potential Anticancer Drugs.Mar Drugs. 2020 Sep 19;18(9):476. doi: 10.3390/md18090476. Mar Drugs. 2020. PMID: 32961827 Free PMC article. Review.
-
Modulation of the ubiquitin-proteasome system by marine natural products.Redox Biol. 2021 May;41:101897. doi: 10.1016/j.redox.2021.101897. Epub 2021 Feb 17. Redox Biol. 2021. PMID: 33640701 Free PMC article. Review.
-
A marine fungus-derived nitrobenzoyl sesquiterpenoid suppresses receptor activator of NF-κB ligand-induced osteoclastogenesis and inflammatory bone destruction.Br J Pharmacol. 2020 Sep;177(18):4242-4260. doi: 10.1111/bph.15179. Epub 2020 Aug 11. Br J Pharmacol. 2020. PMID: 32608081 Free PMC article.
-
Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities.Mar Drugs. 2021 Aug 20;19(8):467. doi: 10.3390/md19080467. Mar Drugs. 2021. PMID: 34436306 Free PMC article.
References
-
- Mayer A.M., Rodriguez A.D., Berlinck R.G., Fusetani N. Marine pharmacology in 2009–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs. 2013;11:2510–2573. doi: 10.3390/md11072510. - DOI - PMC - PubMed
-
- Mayer A.M., Rodriguez A.D., Berlinck R.G., Fusetani N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis and antiviral activities; affecting the immune and nervous system and other miscellaneous mechanism of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011;153:191–222. doi: 10.1016/j.cbpc.2010.08.008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

