Corticosteroid therapy for nephrotic syndrome in children
- PMID: 25785660
- PMCID: PMC7025788
- DOI: 10.1002/14651858.CD001533.pub5
Corticosteroid therapy for nephrotic syndrome in children
Update in
-
Corticosteroid therapy for nephrotic syndrome in children.Cochrane Database Syst Rev. 2020 Aug 31;2020(8):CD001533. doi: 10.1002/14651858.CD001533.pub6. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2024 Aug 22;8:CD001533. doi: 10.1002/14651858.CD001533.pub7. PMID: 35659203 Free PMC article. Updated.
Abstract
Background: In nephrotic syndrome protein leaks from the blood to the urine through the glomeruli resulting in hypoproteinaemia and generalised oedema. While most children with nephrotic syndrome respond to corticosteroids, 80% experience a relapsing course. Corticosteroids have reduced the mortality rate to around 3%. However corticosteroids have well recognised potentially serious adverse effects such as obesity, poor growth, hypertension, diabetes mellitus, osteoporosis and behavioural disturbances. This is an update of a review first published in 2000 and updated in 2003, 2005 and 2007.
Objectives: The aim of this review was to assess the benefits and harms of different corticosteroid regimens in children with steroid-sensitive nephrotic syndrome (SSNS). The benefits and harms of therapy were studied in two groups of children 1) children in their initial episode of SSNS, and 2) children who experience a relapsing course of SSNS.
Search methods: We searched the Cochrane Renal Group's Specialised Register to 26 February 2015 through contact with the Trials Search Co-ordinator using search terms relevant to this review.
Selection criteria: Randomised controlled trials (RCTs) performed in children (three months to 18 years) in their initial or subsequent episode of SSNS, comparing different durations, total doses or other dose strategies using any corticosteroid agent.
Data collection and analysis: Two authors independently assessed risk of bias and extracted data. Results were expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
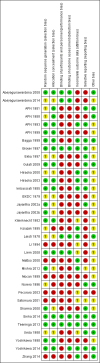
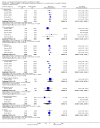
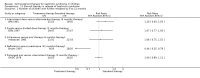
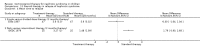
Main results: Ten new studies were identified so a total of 34 studies (3033 total participants) were included in the 2015 review update. The risk of bias attributes were frequently poorly performed. Low risk of bias was reported in 18 studies for sequence generation, 16 studies for allocation concealment, seven for performance and detection bias, 15 for incomplete reporting and 16 for selective reporting. Three months or more of prednisone significantly reduced the risk of frequently relapsing nephrotic syndrome (FRNS) (6 studies, 582 children: RR 0.68, 95% CI 0.47 to 1.00) and of relapse by 12 to 24 months (8 studies, 741 children: RR 0.80, 95% CI 0.64 to 1.00) compared with two months. Five or six months of prednisone significantly reduced the risk of relapse (7 studies, 763 children: RR 0.62, 95% CI 0.45 to 0.85) but not FRNS (5 studies, 591 children: RR 0.78, 95% CI 0.50 to 1.22) compared with three months. However there was significant heterogeneity in the analyses. Subgroup analysis stratified by risk of bias for allocation concealment showed that the risk for FRNS did not differ significantly between two or three months of prednisone and three to six months among studies at low risk of bias but was significantly reduced in extended duration studies compared with two or three months in studies at high risk or unclear risk of bias. There were no significant differences in the risk of adverse effects between extended duration and two or three months of prednisone. Four studies found that in children with FRNS, daily prednisone during viral infections compared with alternate-day prednisone or no treatment significantly reduced the rate of relapse.
Authors' conclusions: In this 2015 update the addition of three well-designed studies has changed the conclusion of this review. Studies of long versus shorter duration of corticosteroids have heterogeneous treatment effects, with the older high risk of bias studies tending to over-estimate the effect of longer course therapy, compared with more recently published low risk of bias studies. Among studies at low risk of bias, there was no significant difference in the risk for FRNS between prednisone given for two or three months and longer durations or total dose of therapy indicating that there is no benefit of increasing the duration of prednisone beyond two or three months in the initial episode of SSNS.The risk of relapse in children with FRNS is reduced by the administration of daily prednisone at onset of an upper respiratory tract or viral infection. Three additional studies have increased the evidence supporting this conclusion. This management strategy may be considered for children with FRNS. A paucity of data on prednisone use in relapsing nephrotic syndrome remains. In particular there are no data from RCTs evaluating the efficacy and safety of prolonged courses of low dose alternate-day prednisone although this management strategy is recommended in current guidelines.
Conflict of interest statement
Deirdre Hahn: none known
Elisabeth Hodson: none known
Narelle Willis: none known
Jonathan Craig: none known
Figures

















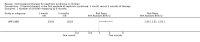

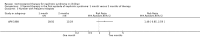

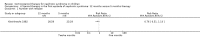
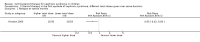
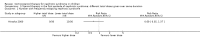























Update of
-
Corticosteroid therapy for nephrotic syndrome in children.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD001533. doi: 10.1002/14651858.CD001533.pub4. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2015 Mar 18;(3):CD001533. doi: 10.1002/14651858.CD001533.pub5. PMID: 17943754 Updated.
References
References to studies included in this review
Abeyagunawardena 2008 {published data only}
-
- Abeyagunawardena AS, Trompeter RS. Increasing the dose of prednisolone during viral infections reduces the risk of relapse in nephrotic syndrome: a randomised controlled trial. Archives of Disease in Childhood 2008;93(3):226‐8. [MEDLINE: ] - PubMed
Abeyagunawardena 2014 {published and unpublished data}
-
- Abeyagunawardena A, Thalgahagoda S, Illangasekera Y, Trompeter R. A short course of prednisolone during an upper respiratory tract infection reduces the risk of relapse in childhood nephrotic syndrome [abstract]. Pediatric Nephrology 2014;29(9):1689. [EMBASE: 71662409] - PubMed
-
- Abeyagunawardena AS, Thalgahagoda S, Illangasekera Y, Trompeter RS. Short course of daily prednisolone during upper respiratory tract infection reduces the risk of relapse in childhood nephrotic syndrome [abstract]. Archives of Disease in Childhood 2014;99:A155‐6. [EMBASE: 71566356]
APN 1981 {published data only}
-
- Anonymous. Alternate‐day prednisone is more effective than intermittent prednisone in frequently relapsing nephrotic syndrome. A report of "Arbeitsgemeinschaft fur Padiatrische Nephrologie". European Journal of Pediatrics 1981;135(3):229‐37. [MEDLINE: ] - PubMed
-
- Anonymous. Alternate‐day versus intermittent prednisone in frequently relapsing nephrotic syndrome. A report of "Arbetsgemeinschaft fur Padiatrische Nephrologie". Lancet 1979;1(8113):401‐3. [MEDLINE: ] - PubMed
-
- Brodehl J, Krohn HP. Steroid trial in frequently relapsing nephrotic syndrome in children. Glomerulonephritis. International Conference on pathogenesis, pathology and treatment. New York: Wiley, 1977:210‐5.
APN 1988 {published data only}
-
- Anonymous. Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Lancet 1988;1(8582):380‐3. [MEDLINE: ] - PubMed
-
- Brodehl J, Krohn HP, Ehrich JH. The treatment of minimal change nephrotic syndrome (lipoid nephrosis): cooperative studies of the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Klinische Padiatrie 1982;194(3):162‐5. [MEDLINE: ] - PubMed
-
- Ehrich JH. Initial treatment of idiopathic nephrotic syndrome: short vs standard prednisone [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:59. [CENTRAL: CN‐00644361]
-
- Ehrich JH for the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Short initial prednisone therapy versus standard prednisone therapy in the steroid responsive nephrotic syndrome [abstract]. Pediatric Nephrology 1987;1(1):C28. [CENTRAL: CN‐00445202]
APN 1993 {published data only}
-
- Ehrich JH, Arbeitsgemeinschaft fur Padiatrische Nephrologie. Minimal change nephrotic syndrome: long prednisone therapy vs standard prednisone therapy [abstract]. Nephrology Dialysis Transplantation 1991;6(10):771. [CENTRAL: CN‐00260613]
-
- Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft fur Padiatrische Nephrologie. European Journal of Pediatrics 1993;152(4):357‐61. [MEDLINE: ] - PubMed
APN 1999 {published data only}
-
- Arbeitsgemeinschaft fur Padiatrische Nephrologie. Results of the nephrotic syndrome study VIII of the APN: new standard treatment versus new standard treatment plus 8 weeks cyclosporin A [abstract]. Pediatric Nephrology 1999;13:C26. [CENTRAL: CN‐00636143]
-
- Hoyer PF. Results of the nephrotic syndrome study VIII of the APN: new standard treatment versus new standard treatment plus 8 weeks cyclosporin A [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):104A. [CENTRAL: CN‐00550522]
-
- Hoyer PF for the Arbeitsgemeinschaft fur Padiatrische Nephrologie. The initial treatment of idiopathic nephrotic syndrome with prednisone and cyclosporin A: preliminary results of a therapeutic trial [abstract]. Pediatric Nephrology 1995;9(6):C91. [CENTRAL: CN‐00401335]
-
- Hoyer PF, Brodehl J. Initial treatment of idiopathic nephrotic syndrome in children: prednisone versus prednisone plus cyclosporine A: a prospective, randomized trial. Journal of the American Society of Nephrology 2006;17(4):1151–7. [MEDLINE: ] - PubMed
Bagga 1999 {published and unpublished data}
-
- Bagga A, Hari P, Srivastava RN. Long (LP) versus standard (SP) initial prednisolone treatment for idiopathic nephrotic syndrome (NS) [abstract no: P225]. Pediatric Nephrology 1998;12:C155. [CENTRAL: CN‐00583944]
-
- Bagga A, Hari P, Srivastava RN. Long (LP) versus standard (SP) initial prednisolone treatment for nephrotic syndrome (NS) [abstract]. 3rd Congress. Nephrology Urology Transplantation Society (NUTS) of SAARC; 1999 Feb 18 ‐ 21; Colombo, Sri Lanka. 1999:158. [CENTRAL: CN‐00460325]
-
- Bagga A, Hari P, Srivastava RN. Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatric Nephrology 1999;13(9):824‐7. [MEDLINE: ] - PubMed
Broyer 1997 {published data only}
-
- Broyer M, Terzi F, Gagnadoux MF, Guest G, Niaudet P. A randomized double blind study of deflazacort (D) versus prednisone (P) in the treatment of idiopathic nephrotic syndrome (INS) [abstract]. Journal of the American Society of Nephrology 1995;6(3):414. [CENTRAL: CN‐00483349]
-
- Broyer M, Terzi F, Lehnert A, Gagnadoux MF, Guest G, Niaudet P. A controlled study of deflazacort in the treatment of idiopathic nephrotic syndrome. Pediatric Nephrology 1997;11(4):418‐22. [MEDLINE: ] - PubMed
Ekka 1997 {published data only}
-
- Bagga A, Ekka BK, Srivastava RN. Single versus divided dose prednisolone therapy for relapses of nephrotic syndrome (NS) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A113. [CENTRAL: CN‐00261391] - PubMed
-
- Ekka BK, Bagga A, Srivastava RN. Single‐ versus divided‐dose prednisolone therapy for relapses of nephrotic syndrome. Pediatric Nephrology 1997;11(5):597‐9. [MEDLINE: ] - PubMed
Gulati 2009 {published data only}
-
- Gulati A, Math A, Sreeniwas V, Hari P, Bagga A. Administration of daily corticosteroids prevents infection associated relapses in frequently relapsing nephrotic syndrome [abstract no: SU714]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
Hiraoka 2000 {published data only}
-
- Hiraoka M, Sudo M, West Japanese Cooperative Study of Kidney Disease in Children. Low versus standard dosage of prednisolone for initial treatment of idiopathic nephrotic syndrome in children [abstract no: A0445]. Journal of the American Society of Nephrology 1996;7(9):1335.
-
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from higher initial prednisolone therapy for nephrotic syndrome. The West Japan Cooperative Study of Kidney Disease in Children. Kidney International 2000;58(3):1247‐52. [MEDLINE: ] - PubMed
-
- Hiraoka M, Tsukahara H, Haruki S, Hayashi S, Takeda N, Miyagawa K, et al. Older boys benefit from intensive initial prednisolone therapy for nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):103A. [CENTRAL: CN‐00550605]
Hiraoka 2003 {published data only}
-
- Hiraoka M, Tsukahara H, Matsubara K, Tsurusawa M, Takeda N, Haruki S, et al. A randomized study of two long‐course prednisolone regimens for nephrotic syndrome in children. American Journal of Kidney Diseases 2003;41(6):1155‐62. [MEDLINE: ] - PubMed
Imbasciati 1985 {published data only}
ISKDC 1979 {published data only}
-
- Anonymous. Nephrotic syndrome in children: a randomized trial comparing two prednisone regimens in steroid‐responsive patients who relapse early. Report of the International Study of Kidney Disease in Children. Journal of Pediatrics 1979;95(2):239‐43. [MEDLINE: ] - PubMed
Jayantha 2002a {published and unpublished data}
-
- Jayantha UK. Comparison of ISKDC regime with a 7 months steroid regime in the first attack of nephrotic syndrome [abstract]. Pediatric Nephrology 2004;19:C81. [CENTRAL: CN‐00583710]
-
- Jayantha UK. Comparison of ISKDC regime with a six month steroid regime in the treatment of steroid sensitive nephrotic syndrome. Unpublished results 2002.
-
- Jayantha UK. Comparison of ISKDC regime with a six month steroid regime in the treatment of steroid sensitive nephrotic syndrome [abstract no: FP2B]. 7th Asian Congress of Pediatric Nephrology; 2000 Nov 1‐4; Singapore. 2000:28. [CENTRAL: CN‐00583708]
Jayantha 2002b {published and unpublished data}
-
- Jayantha UK. Comparison of ISKDC regime with 6 month regime in patients with relapsing nephrotic syndrome. Unpublished results 2002.
-
- Jayantha UK. Prolong versus standard steroid therapy for children with relapsing course of nephrotic syndrome [abstract no: P026]. Pediatric Nephrology 2004;19(9):C99. [MEDLINE: ]
Kleinknecht 1982 {published and unpublished data}
-
- Kleinknecht C, Broyer M, Parchoux B, Loriat C, Nivet H, Palcoux JB, et al. Comparison of short and long treatment at onset of steroid sensitive nephrosis (SSN). Preliminary results of a multicenter controlled trial for the French Society of Pediatric Nephrology [abstract]. International Journal of Pediatric Nephrology 1982;3(1):45. [CENTRAL: CN‐00550480]
Ksiazek 1995 {published data only}
-
- Ksiazek J, Wyszynska T. Short versus long initial prednisone treatment in steroid‐sensitive nephrotic syndrome in children. Acta Paediatrica 1995;84(8):889‐93. [MEDLINE: ] - PubMed
Leisti 1978 {published data only}
Li 1994 {published data only}
-
- Li X, Li Z, Cheng Z. Treatment of children with simple nephrotic syndrom using prednison once per day. Acta Academiae Medicinae Hubei 1994;15(4):386‐8. [EMBASE: 1995005617]
Liern 2008 {published data only}
-
- Liern M, Dieguez S, Canepa C. Recovery of total immunoglobulin and immunoglobulin subclasses in nephrotic syndrome: deflazacort vs methylprednisone [Recuperacion de la inmnoglobulina total y sus subclases en el sindrome nefrotico: deflazacort vs metilprednisona]. Nefrologia 2008;28(5):563. [MEDLINE: ] - PubMed
Mattoo 2000 {published data only}
-
- Mattoo TK, Mahmoud MA. Increased maintenance corticosteroids during upper respiratory infection decrease the risk of relapse in nephrotic syndrome. Nephron 2000;85(4):343‐5. [MEDLINE: ] - PubMed
Mishra 2012 {published data only}
-
- Mishra OP, Thakur N, Mishra RN, Prasad R. Prolonged versus standard prednisolone therapy for initial episode of idiopathic nephrotic syndrome. Journal of Nephrology 2012;25(3):394‐400. [MEDLINE: ] - PubMed
Mocan 1999 {published data only}
-
- Mocan H, Erduran E, Karaguzel G. High dose methylprednisolone therapy in nephrotic syndrome. Indian Journal of Pediatrics 1999;66(2):171‐4. [MEDLINE: ] - PubMed
-
- Mocan H, Mocan MZ, Erduran E, Karaguzel G, Aslan Y. The effect of high dose methylprednisolone therapy in patients with minimal change nephrotic syndrome [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A74. [CENTRAL: CN‐00261380]
Norero 1996 {published data only}
-
- Norero C, Delucchi A, Lagos E, Rosati P. Initial therapy of primary nephrotic syndrome in children: evaluation in a period of 18 months of two prednisone treatment schedules. Chilean Co‐operative Group of Study of Nephrotic Syndrome in Children [Cuadro inicial del sindrome nefrosico primario del nino: evaluacion a 18 meses de dos esquemas de tratamiento con prednisona. Groupo Cooperativo Chileno de Estudio del Sindrome Nefrosico del Nino]. Revista Medica de Chile 1996;124(5):567‐72. [MEDLINE: ] - PubMed
-
- Norero C, Delucchi A, Lagos E, Rosati P. Long term evaluation of two prednisone treatments in initial idiopathic nephrotic syndrome (INS) in children. Preliminary findings [abstract]. Pediatric Nephrology 1995;9(6):C88. [CENTRAL: CN‐00550669]
Pecoraro 2003 {published data only}
-
- Pecoraro C, Caropreso MR, Malgieri G, Ferretti A, Nuzzi F. Therapy of first episode steroid responsive nephrotic syndrome (FESRNS): a randomised controlled trial [abstract no: MP087]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v230. [CENTRAL: CN‐00644161]
-
- Pecoraro C, Caropreso MR, Malgieri G, Ferretti AVS, Raddi G, Piscitelli A, et al. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial [abstract no: OFC41]. Pediatric Nephrology 2004;19(9):C72. [CENTRAL: CN‐00644160]
-
- Pecoraro C, Caropreso MR, Passaro G, Ferretti AVS, Malgieri G. Therapy of first episode of steroid responsive nephrotic syndrome: a randomised controlled trial [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):63. [CENTRAL: CN‐00447140]
Satomura 2001 {published data only}
-
- Satomura K, Yamaoka K, Shima M, Tanaka Y, Ashino N, Nakagawa K, et al. Standard vs low initial dose of prednisolone therapy for first episodes of nephrotic syndrome in children [abstract]. Pediatric Nephrology 2001;16(8):C117. [CENTRAL: CN‐00447593]
Sharma 2000 {unpublished data only}
-
- Gulati S, Ahmed M, Sharma RK, Gupta A, Pokhariyal S. Comparison of abrupt withdrawal versus slow tapering regimen of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A87. [CENTRAL: CN‐00445595]
-
- Sharma RK, Ahmed M, Gulati S, Gupta A, Pokhariyal S. Comparison of abrupt withdrawal versus slow tapering regimen of prednisolone therapy in the management of first episode of steroid responsive childhood idiopathic nephrotic syndrome. Unpublished results 2002.
-
- Sharma RK, Ahmed M, Gupta A, Gulati S, Sharma AP. Comparison of abrupt withdrawal versus slow tapering regimens of prednisolone therapy in management of first episode of steroid responsive childhood idiopathic nephrotic syndrome [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):97A. [CENTRAL: CN‐00550434]
Sinha 2014 {published and unpublished data}
-
- Bagga A, Sinha A, Saha A, Kumar M, Afzal K, Mehta A, et al. Randomized double blind, placebo controlled trial to compare the efficacy of 3‐months versus 6‐months therapy with prednisolone for the first episode of idiopathic nephrotic syndrome (CTRI/2010/091/001095) [abstract]. Pediatric Nephrology 2012;27(9):1707. [EMBASE: 71386357]
-
- Sinha A, Bagga A, Sharma S, Saha A, Kumar M, Afzal K, et al. Randomized, double blind, placebo controlled trial to compare the efficacy of 3‐months versus 6‐months therapy with prednisolone for the first episode of idiopathic nephritic syndrome [abstract]. Pediatric Nephrology 2013;28(8):1361‐2. [EMBASE: 71126981]
-
- Sinha A, Saha A, Kumar M, Sharma S, Afzal K, Mehta A, et al. Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid‐ sensitive nephrotic syndrome. Kidney International 2014;87(1):217‐24. [MEDLINE: ] - PubMed
Teeninga 2013 {published data only}
-
- Teeninga N, Kist‐Van Holthe JE, Ruswuk N, Mos NI, Hop WC, Wetzels JF, et al. Effect of extended prednisolone treatment from three to six months, with equal cumulative doses, on childhood nephrotic syndrome: a nation‐wide randomised controlled trial [abstract]. Pediatric Nephrology 2012;27(9):1637‐8. [EMBASE: 71386208]
-
- Teeninga N, Kist‐van Holthe JE, Akker EL, Kersten MC, Boersma E, Krabbe HG, et al. Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney International 2014;85(6):1444‐53. [MEDLINE: ] - PubMed
Ueda 1988 {published data only}
-
- Ueda N, Chihara M, Kawaguchi S, Niimomi Y, Nonada T, Matsumoto J, et al. Intermittent versus long‐term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. Journal of Pediatrics 1988;112(1):122‐6. [MEDLINE: ] - PubMed
Yoshikawa 1998 {published data only}
-
- Takekoshi Y. Treatment of idiopathic nephrotic syndrome [abstract]. Pediatric Nephrology 1996;10(1):C3. [CENTRAL: CN‐00402800]
-
- Yoshikawa N, Ito H, Takehoshi Y, Honda M, Awazu M, Iijima K, et al. Standard versus long‐term prednisolone with Sairei‐to for initial therapy in childhood steroid‐responsive nephrotic syndrome: a prospective controlled study. Nippon Jinzon Gakkai Shi. Japanese Journal of Nephrology 1998;40(8):587‐90. [MEDLINE: ] - PubMed
Yoshikawa 2014 {published and unpublished data}
-
- Yoshikawa N, Nakanishi K, Oba MS, Sako M, Ohashi Y, Iijima K. Increased duration and dose of prednisolone (PSL) treatment does not reduce relapses in childhood nephrotic syndrome [abstract no: SA‐PO1080]. Journal of the American Society of Nephrology 2014;24(Abstracts):3B.
Zhang 2014 {published data only}
-
- Zhang B, Liu T, Wang W, Zhang X, Fan S, Liu Z, et al. A prospective randomly controlled clinical trial on azithromycin therapy for induction treatment of children with nephrotic syndrome. European Journal of Pediatrics 2014;173(4):509‐15. [MEDLINE: ] - PubMed
References to studies excluded from this review
Alatas 1978 {published data only}
-
- Alatas H, Wirya IG, Tambunan T, Himawan S. Controlled trial of chlorambucil in frequently relapsing nephrotic syndrome in children (a preliminary report). Journal of the Medical Association of Thailand 1978;61 Suppl 1:222‐8. [MEDLINE: ] - PubMed
Anonymous 1968 {published data only}
-
- Anonymous. Nephrotic syndrome in children [Die nefrotiese sindroom by kinders]. South African Medical Journal. Suid‐Afrikaanse Tydskrif Vir Geneeskunde 1968;42(2):21‐2. [MEDLINE: ] - PubMed
APN 1982 {published data only}
-
- Anonymous. Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. New England Journal of Medicine 1982;306(8):451‐4. [MEDLINE: ] - PubMed
-
- Brodehl J, Krohn HP, Ehrich JH. The treatment of minimal change nephrotic syndrome (lipoid nephrosis): cooperative studies of the Arbeitsgemeinschaft fur Padiatrische Nephrologie (APN). Klinische Padiatrie 1982;194(3):162‐5. [MEDLINE: ] - PubMed
Arun 2009 {published data only}
-
- Arun S, Bagga A, Bhatnagar S, Hari P, Menon S, Saini S. Efficacy of Zinc(Zn) in reducing relapses in steroid sensitive nephrotic syndrome (SSNS) double blind, randomized controlled trial (RCT) (CRG030600044) [abstract no: 184OP]. Pediatric Nephrology 2007;22(9):1481. [CENTRAL: CN‐00724915] - PubMed
-
- Arun S, Bhatnagar S, Menon S, Saini S, Hari P, Bagga A. Efficacy of zinc supplements in reducing relapses in steroid‐sensitive nephrotic syndrome. Pediatric Nephrology 2009;24(8):1583‐6. [MEDLINE: ] - PubMed
Baluarte 1978 {published data only}
-
- Baluarte HJ, Hiner L, Gruskin AB. Chlorambucil dosage in frequently relapsing nephrotic syndrome: a controlled clinical trial. Journal of Pediatrics 1978;92(2):295‐8. [MEDLINE: ] - PubMed
BAPN 1991 {published data only}
-
- Anonymous. Levamisole for corticosteroid‐dependent nephrotic syndrome in childhood. British Association for Paediatric Nephrology. Lancet 1991;337(8757):1555‐7. [MEDLINE: ] - PubMed
Barratt 1970 {published data only}
-
- Barratt TM, Soothill JF. A controlled trial of cyclophosphamide in steroid sensitive relapsing nephrotic syndrome of childhood [abstract]. Nephron 1971;8:95. [CENTRAL: CN‐00253521] - PubMed
-
- Barratt TM, Soothill JF. Controlled trial of cyclophosphamide in steroid‐sensitive relapsing nephrotic syndrome of childhood. Lancet 1970;2(7671):479‐2. [MEDLINE: ] - PubMed
Barratt 1973 {published data only}
Barratt 1977 {published data only}
Cerkauskiene 2005 {published data only}
-
- Cerkauskiene R, Kaltenis P. Comparative study of prednisolone alone and prednisolone plus fusidic acid in the treatment of children with steroid‐responsive nephrotic syndrome. Medicina (Kaunas, Lithuania) 2005;41 Suppl 1:26‐30. [MEDLINE: ] - PubMed
Chiu 1973 {published data only}
-
- Chiu J, McLaine PN, Drummond KN. A controlled prospective study of cyclophosphamide in relapsing, corticosteroid‐responsive, minimal‐lesion nephrotic syndrome in childhood. Journal of Pediatrics 1973;82(4):607‐13. [MEDLINE: ] - PubMed
Dayal 1994 {published data only}
-
- Dayal U, Dayal AK, Shastry JC, Raghupathy P. Use of levamisole in maintaining remission in steroid‐sensitive nephrotic syndrome in children [erratum appears in Nephron 1994;67(4):507]. Nephron 1994;66(4):408‐12. [MEDLINE: ] - PubMed
Donia 2005 {published data only}
-
- Donia A, Ammar H, Moustafa F, Sobh M. Long‐term efficacy of two unconventional adjunctive therapies in minimal change nephrotic children [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:37. [CENTRAL: CN‐00509161]
-
- Donia AF, Ammar HM, Agroudy A, Moustafa F, Sobh MA. Long‐term results of two unconventional agents in steroid‐dependent nephrotic children. Pediatric Nephrology 2005;20(10):1420‐5. [MEDLINE: ] - PubMed
Edefonti 1988 {published data only}
-
- Edefonti A, Ghio L, Bettinelli A, Paterlini G, Giani M, Nebbia G, et al. Unconjugated hyperbilirubinemia due to ciclosporin administration in children with nephrotic syndrome. Contributions to Nephrology 1988;67:121‐4. [MEDLINE: ] - PubMed
-
- Edefonti A, Ghio L, Bettinelli G, Paterlini M, Giani G Nebbia A, et al. Unconjugated hyperbilirubinemia due to cyclosporin A (CYA) administration in children with nephrotic syndrome [abstract]. Pedatric Nephrology 1987;1(1):C8. [CENTRAL: CN‐00465774] - PubMed
-
- Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, et al. Cyclosporine (CSA) vs cyclophosphamide (CYC) for children with frequently relapsing/steroid dependent nephrotic syndrome (FR/SDNS): long term study [abstract]. Journal of the American Society of Nephrology 1992;3(3):310.
-
- Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, Lama G, et al. Cyclosporine (CSA) vs cyclophosphamide (CYC) for children with frequently relapsing/steroid dependent nephrotic syndrome: long term study [abstract]. 9th Congress of the International Pediatric Nephrology Association; 1992 Aug 30‐Sep 4; Jerusalem (Israel). 1992:C70. [CENTRAL: CN‐00483820]
-
- Ponticelli C. A multicenter controlled prospective trial with cyclosporine vs cyclophosphamide in frequent relapsers and steroid dependent patients with idiopathic nephrotic syndrome. Journal of Nephrology 1989;2(2):147‐51. [EMBASE: 1991014828]
Grupe 1976 {published data only}
-
- Grupe WE, Makker SP, Ingelfinger JR. Chlorambucil treatment of frequently relapsing nephrotic syndrome. New England Journal of Medicine 1976;295(14):746‐9. [MEDLINE: ] - PubMed
Hu 2006 {published data only}
-
- Hu GH, Dang XQ, Wang JH. Clinical effect of shenbing mistura combined with glucocorticoid on recurrent nephrotic syndrome in children and levels of interleukin‐6 and tumor necrosis factor‐alpha in blood and urine. Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional & Western Medicine] 2006;26(10):892‐5. [MEDLINE: ] - PubMed
Idczak‐Nowicka 1996 {published data only}
-
- Idczak‐Nowicka E, Ksiazek J, Krynski J, Wyszynska T. Verification of indications for kidney biopsy in children with steroid‐dependent nephrotic syndrome [Weryfikacja wskazan do biopsji nerki u dzieci ze sterydozaleznym zespolem nerczycowym]. Pediatria Polska 1996;71(8):679‐83. [MEDLINE: ] - PubMed
ISKDC 1970 {published data only}
-
- Abramowicz M, Barnett HL, Edelmann CM Jr, Griefer I, Kobayashi O, Arneil GC, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970;1(7654):959‐61. [MEDLINE: ] - PubMed
-
- Arneil GC. International trial of azathioprine in nephrotic syndrome in childhood. Nephron 1971;8:95. [CENTRAL: CN‐00253520]
ISKDC 1974 {published data only}
-
- Anonymous. Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Report of the International study of Kidney Disease in Children. Lancet 1974;2(7878):423‐7. [MEDLINE: ] - PubMed
Liu 1995 {published data only}
-
- Liu XY. Therapeutic effect of chai‐ling‐tang (sairei‐to) on the steroid‐dependent nephrotic syndrome in children. American Journal of Chinese Medicine 1995;23(3‐4):255‐60. [MEDLINE: ] - PubMed
Martinelli 2004 {published data only}
-
- Martinelli R, Pereira LJ, Silva OM, Okumura AS, Rocha H. Cyclophosphamide in the treatment of focal segmental glomerulosclerosis. Brazilian Journal of Medical & Biological Research 2004;37(9):1365‐72. [MEDLINE: ] - PubMed
McCrory 1973 {published data only}
-
- McCrory WW, Shibuya M, Lu WH, Lewy JE. Therapeutic and toxic effects observed with different dosage programs of cyclophosphamide in treatment of steroid‐responsive but frequently relapsing nephrotic syndrome. Journal of Pediatrics 1973;82(4):614‐8. [MEDLINE: ] - PubMed
Niaudet 1992 {published data only}
-
- Niaudet P. Comparison of cyclosporin and chlorambucil in the treatment of steroid‐dependent idiopathic nephrotic syndrome: A multicentre randomized controlled trial. The French Society of Paediatric Nephrology. Pediatric Nephrology 1992;6(1):1‐3. [MEDLINE: ] - PubMed
Prasad 2004 {published data only}
-
- Prasad N, Gulati S, Sharma RK, Singh U, Ahmed M. Pulse cyclophosphamide therapy in steroid‐dependent nephrotic syndrome. Pediatric Nephrology 2004;19(5):494‐8. [MEDLINE: ] - PubMed
Rashid 1996 {published data only}
-
- Rashid HU, Ahmed S, Fatima N, Khanam A. Levamisole in the treatment of steroid dependent or frequent relapsing nephrotic syndrome in children. Bangladesh Renal Journal 1996;15(1):6‐8. [EMBASE: 1996266847]
Sharipov 2007 {published data only}
-
- Sharipov AM, Khamzaeva KA. Current aspects of transformation of morphological changes in children with nephrotic syndrome depending on the treatment regimen. Urologiia (Moscow, Russia) 2007, (2):68‐71. [MEDLINE: ] - PubMed
Ueda 1990 {published data only}
Wang 2005 {published data only}
Weiss 1993 {published data only}
-
- Weiss R. Results of a randomized, double‐blind, placebo controlled, multi‐center clinical trial of levamisole for the treatment of children with frequently relapsing or steroid dependent nephrotic syndrome. Personal communication 2005.
-
- Weiss R, NY‐NJ‐Phila‐Pediatric Nephrology Study Group. Randomized, double‐blind, placebo (P) controlled trial of levamisole (L) for children (CH) with frequently relapsing/steroid dependant (FR/SD) nephrotic syndrome (NS) [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):289. [CENTRAL: CN‐00520404]
Wingen 1990 {published data only}
-
- Wingen AM, Muller‐Wiefel DE, Scharer K. Comparison of different regimens of prednisone therapy in frequently relapsing nephrotic syndrome. Acta Paediatrica Scandinavica 1990;79(3):305‐10. [MEDLINE: ] - PubMed
Yamashita 1971 {published data only}
-
- Yamashita F, Funatsu T, Nagayama K, Arihiro H, Anan S. Evaluation of alternate‐day steroid therapy for nephrotic syndrome in childhood by cross‐over study. Kurume Medical Journal 1971;18(3):153‐60. [MEDLINE: ] - PubMed
Yang 2001 {published data only}
-
- Yang RY, Xu LF, Wang Q. The effect of Xuezhikang on lipid metabolism in patients with nephrotic syndrome. Hubei Yikedaxue Xuebao 2001;22(1):55‐7.
Yoshioka 2000 {published data only}
-
- Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney International 2000;58(1):317‐24. [MEDLINE: ] - PubMed
-
- Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. Placebo‐controlled trial of mizoribine (MZR) in children with frequently relapsing nephrotic syndrome (FRNS). The Pediatric Mizoribine Study Group [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):163A.
References to ongoing studies
PREDNOS 2 Study 2014 {published data only}
-
- Webb NJ, Frew E, Brettell EA, Milford DV, Bockenhauer D, Saleem MA, et al. Short course daily prednisolone therapy during an upper respiratory tract infection in children with relapsing steroid‐sensitive nephrotic syndrome (PREDNOS 2): protocol for a randomised controlled trial. Trials 2014;15:147. [MEDLINE: ] - PMC - PubMed
PREDNOS Study 2013 {published data only}
-
- Webb N, Trompeter R, Cummins C, Wheatley K, Frew E. Long‐term tapering versus standard prednisolone (steroid) therapy for the treatment of the initial episode of childhood nephrotic syndrome: national multicentre randomised double‐blind trial. www.controlled‐trials.com/ISRCTN16645249 (accessed 26 February 2015).
Additional references
Arneil 1971
-
- Arneil GC. The nephrotic syndrome. Pediatric Clinics of North America 1971;18(2):547‐59. [MEDLINE: ] - PubMed
Bhimma 1997
-
- Bhimma R, Coovadia HM, Adhikari M. Nephrotic syndrome in South African children: changing perspectives over 20 years. Pediatric Nephrology 1997;11(4):429‐34. [MEDLINE: ] - PubMed
Bonilla‐Felix 1999
-
- Bonilla‐Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney International 1999;55(5):1885‐90. [MEDLINE: ] - PubMed
Eddy 2003
-
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet 2003;362(9384):629‐39. [MEDLINE: ] - PubMed
Egger 2001
-
- Egger M, Dickersin K, Davey Smith G. Problems and limitations in conducting systematic reviews. In: Egger M, Davey Smith G, Altman D editor(s). Systematic Reviews in Health care: Meta‐analysis in context. Second Edition. London: BMJ Publishing Group, 2001.
El Bakkali 2011
Elzouki 1984
Gipson 2009
-
- Gipson DS, Massengill SF, Yoa L, Nagaraj S, Smoyer WE, Mahan JD, et al. Management of childhood onset nephrotic syndrome. Pediatrics 2009;124(2):747‐57. [MEDLINE: ] - PubMed
Gipson 2011
Greenbaum 2012
-
- Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome‐‐current and future therapies. Nature Reviews Nephrology 2012;8(8):445‐58. [MEDLINE: ] - PubMed
Gulati 1999
-
- Gulati A, Sharma AP, Sharma RK, Gupta A. Changing trends of histopathology in childhood nephrotic syndrome. American Journal of Kidney Diseases 1999;34(4):159‐65. [MEDLINE: ] - PubMed
Haute Autorité de Santé 2008
-
- Haute Autorité de Santé. [Syndrome néphrotique idiopathique de l'enfant. Protocole national de diagnostic et de soinspour une maladie rare. 2008; 1‐22]. www.has‐sante.fr/portail/upload/docs/application/pdf/2008‐06/pnds_sni_enfant.pdf (accessed 26 February 2015).
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopewell 2007
Hyams 1988
-
- Hyams JS, Carey DE. Corticosteroids and growth. Journal of Pediatrics 1988;113(2):249‐54. [MEDLINE: ] - PubMed
IPNG‐IAP 2008
-
- Indian Pediatric Nephrology Group, Indian Academy of Pediatrics, Bagga A, Ali U, Banerjee S, Kanitkar M, Phadke KD, et al. Management of steroid sensitive nephrotic syndrome: revised guidelines. Indian Pediatrics 2008;45(3):203‐14. [MEDLINE: ] - PubMed
ISKDC 1984
-
- Anonymous. Minimal change nephrotic syndrome in children: deaths during the first 5‐15 years' observation. Report of the International Study of Kidney Disease in Children. Pediatrics 1984;73(4):497‐501. [MEDLINE: ] - PubMed
KDIGO 2012
-
- Lombel RM, Gipson DS, Hodson EM, Kidney Disease: Improving Global Outcomes. Treatment of steroid‐sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatric Nephrology 2013;28(3):415‐26. [MEDLINE: ] - PubMed
Kim 2005
-
- Kim JS, Bellew CA, Silverstein DM, Aviles DH, Boineau FG, Vehaskari VM. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney International 2005;68(3):1275‐81. [MEDLINE: ] - PubMed
Koskimies 1982
MacHardy 2009
-
- MacHardy N, Miles PV, Massengill SF, Smoyer WE, Mahan JD, Greenbaum L, et al. Management patterns of childhood‐onset nephrotic syndrome. Pediatric Nephrology 2009;24(11):2193‐201. [MEDLINE: ] - PubMed
McKinney 2001
-
- McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatric Nephrology 2001;16(12):1040‐4. [MEDLINE: ] - PubMed
Mehls 2011
-
- Mehls O, Hoyer PF. Dosing of glucocorticosteroids in nephrotic syndrome. Pediatric Nephrology 2011;26(12):2095‐8. [MEDLINE: ] - PubMed
Mishra 2010
-
- Mishra OM, Basu B, Upadhyay SK, Prasad R, Schaefer F. Behavioural abnormalities in children with nephrotic syndrome. Nephrology Dialysis Transplantation 2010;25(8):2537‐41. [MEDLINE: ] - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] - PubMed
Moher 2001
-
- Moher D, Jones A, Lepage L, CONSORT Group (Consolidated Standards for Reporting of Trials). Use of the CONSORT statement and quality of reports of randomized trials: a comparative before‐and‐after evaluation. JAMA 2001;285(15):1992‐5. [MEDLINE: ] - PubMed
Neuhaus 2010
-
- Neuhaus TJ, Langlois V, Licht C. Behavioural abnormalities in children with nephrotic syndrome‐‐an underappreciated complication of a standard treatment?. Nephrology Dialysis Transplantation 2010;25(8):2397‐9. [MEDLINE: ] - PubMed
Ng 2001
-
- Ng JS, Wong W, Law RW, Hui J, Wong EN, Lam DS. Ocular complications of paediatric patients with nephrotic syndrome. Clinical & Experimental Ophthalmology 2001;29(4):239‐43. [MEDLINE: ] - PubMed
Niaudet 2009
-
- Niaudet P. Long‐term outcome of children with steroid‐sensitive idiopathic nephrotic syndrome. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(10):1457‐8. [MEDLINE: ] - PubMed
Pravitsitthikul 2013
Rüth 2005
-
- Rüth EM, Kemper M, Leumann EP, Laube GF, Neuhaus TJ. Children with steroid sensitive nephrotic syndrome come of age: long‐term outcome. Journal of Pediatrics 2005;147(2):202‐7. [MEDLINE: ] - PubMed
Samuel 2013
-
- Samuel S, Morgan CJ, Bitzan N, Mammen C, Dart AB, Manns BJ, et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatric Nephrology 2013;29(12):2289‐98. [MEDLINE: ] - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] - PubMed
Srivastava 1999
-
- Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatric Nephrology 1999;13(1):13‐8. [MEDLINE: ] - PubMed
Tarshish 1997
-
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. Journal of the American Society of Nephrology 1997;8(5):769‐76. [MEDLINE: ] - PubMed
References to other published versions of this review
Hodson 2000
Hodson 2002
-
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001533] - DOI
Hodson 2003
Hodson 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

