Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe
- PMID: 25785934
- PMCID: PMC4364720
- DOI: 10.1371/journal.pone.0119551
Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe
Abstract
Background: There is appreciable utilisation of antihistamines (H1) in European countries, either prescribed by physician and purchased by patients for self-medication. Terfenadine and astemizole underwent regulatory restrictions in '90 because of their cardiac toxicity, but only scarce clinical data are available on other antihistamines.
Aim: To investigate the pro-arrhythmic potential of antihistamines by combining safety reports of the FDA Adverse Event Reporting System (FAERS) with drug utilization data from 13 European Countries.
Methods: We identified signals of antihistamine arrhythmogenic potential by analyzing FAERS database for all cases of Torsades de Pointes (TdP), QT abnormalities (QTabn), ventricular arrhythmia (VA) and sudden cardiac death/cardiac arrest (SCD/CA). Number of cases ≥3 and disproportionality were used to define alert signals: TdP and QTabn identified stronger signals, whereas SCD/CA identified weaker signals. Drug utilization data from 2005 to 2010 were collected from administrative databases through health authorities and insurance.
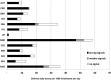
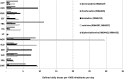
Results: Antihistamines were reported in 109 cases of TdP/QT prolongation, 278 VA and 610 SCD/CA. Five agents resulted in stronger signals (cetirizine, desloratadine, diphenhydramine, fexofenadine, loratadine) and 6 in weaker signals (alimemazine, carbinoxamine, cyclizine, cyproeptadine, dexchlorpheniramine and doxylamine). Exposure to antihistamines with stronger signal was markedly different across European countries and was at least 40% in each Country. Cetirizine was >29 Defined Daily Doses per 1000 inhabitants per day (DID) in Norway, desloratadine >11 DID in France and loratadine >9 DID in Sweden and Croatia. Drugs with weaker signals accounted for no more than 10% (in Sweden) and in most European countries their use was negligible.
Conclusions: Some second-generation antihistamines are associated with signal of torsadogenicity and largely used in most European countries. Although confirmation by analytical studies is required, regulators and clinicians should consider risk-minimisation activities. Also antihistamines without signal but with peculiar use in a few Countries (e.g., levocetirizine) or with increasing consumption (e.g., rupatadine) deserve careful surveillance.
Conflict of interest statement
Figures

Similar articles
-
The Contribution of National Spontaneous Reporting Systems to Detect Signals of Torsadogenicity: Issues Emerging from the ARITMO Project.Drug Saf. 2016 Jan;39(1):59-68. doi: 10.1007/s40264-015-0353-1. Drug Saf. 2016. PMID: 26446144 Free PMC article.
-
Association of H1-antihistamines with torsade de pointes: a pharmacovigilance study of the food and drug administration adverse event reporting system.Expert Opin Drug Saf. 2021 Jan;20(1):101-107. doi: 10.1080/14740338.2021.1846717. Epub 2020 Nov 13. Expert Opin Drug Saf. 2021. PMID: 33141610
-
Comparative analysis of the cardiotoxicity proclivities of second generation antihistamines in an experimental model predictive of adverse clinical ECG effects.Arzneimittelforschung. 1996 Feb;46(2):153-8. Arzneimittelforschung. 1996. PMID: 8720304
-
Pharmacokinetic overview of oral second-generation H1 antihistamines.Int J Clin Pharmacol Ther. 1998 May;36(5):292-300. Int J Clin Pharmacol Ther. 1998. PMID: 9629995 Review.
-
Second-generation antihistamines: the risk of ventricular arrhythmias.Clin Ther. 1999 Feb;21(2):281-95. doi: 10.1016/S0149-2918(00)88286-7. Clin Ther. 1999. PMID: 10211532 Review.
Cited by
-
Wasp sting induced STEMI with complete coronary artery occlusion: a case of Kounis syndrome.BMJ Case Rep. 2017 Sep 7;2017:bcr2017221256. doi: 10.1136/bcr-2017-221256. BMJ Case Rep. 2017. PMID: 28882939 Free PMC article.
-
Time-to-Onset Analysis of Drug-Induced Long QT Syndrome Based on a Spontaneous Reporting System for Adverse Drug Events.PLoS One. 2016 Oct 10;11(10):e0164309. doi: 10.1371/journal.pone.0164309. eCollection 2016. PLoS One. 2016. PMID: 27723808 Free PMC article.
-
The Contribution of National Spontaneous Reporting Systems to Detect Signals of Torsadogenicity: Issues Emerging from the ARITMO Project.Drug Saf. 2016 Jan;39(1):59-68. doi: 10.1007/s40264-015-0353-1. Drug Saf. 2016. PMID: 26446144 Free PMC article.
-
Evaluation of the potential for QTc prolongation with avelumab.Cancer Chemother Pharmacol. 2019 Nov;84(5):1017-1026. doi: 10.1007/s00280-019-03925-z. Epub 2019 Sep 3. Cancer Chemother Pharmacol. 2019. PMID: 31478078 Free PMC article.
-
Effect of QT interval-prolonging drugs taken in pregnancy on the neonatal QT interval.Front Pharmacol. 2023 Aug 7;14:1193317. doi: 10.3389/fphar.2023.1193317. eCollection 2023. Front Pharmacol. 2023. PMID: 37608894 Free PMC article.
References
-
- Grant JA. Molecular pharmacology of second-generation antihistamines. Allergy Asthma Proc 2000; 21:135–140. - PubMed
-
- Tyl B, Kabbaj M, Azzam S, Sologuren A, Valiente R, Reinbolt E et al. Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: results of a thorough QT study (TQTS) with QT-concentration analysis. J Clin Pharmacol 2012; 52:893–903. 10.1177/0091270011407191 - DOI - PubMed
-
- Hulhoven R, Rosillon D, Letiexhe M, Meeus MA, Daoust A, Stockis A. Levocetirizine does not prolong the QT/QTc interval in healthy subjects: results from a thorough QT study. Eur J Clin Pharmacol 2007; 63:1011–1017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

