Myocardial mitochondrial and contractile function are preserved in mice lacking adiponectin
- PMID: 25785965
- PMCID: PMC4364743
- DOI: 10.1371/journal.pone.0119416
Myocardial mitochondrial and contractile function are preserved in mice lacking adiponectin
Abstract
Adiponectin deficiency leads to increased myocardial infarct size following ischemia reperfusion and to exaggerated cardiac hypertrophy following pressure overload, entities that are causally linked to mitochondrial dysfunction. In skeletal muscle, lack of adiponectin results in impaired mitochondrial function. Thus, it was our objective to investigate whether adiponectin deficiency impairs mitochondrial energetics in the heart. At 8 weeks of age, heart weight-to-body weight ratios were not different between adiponectin knockout (ADQ-/-) mice and wildtypes (WT). In isolated working hearts, cardiac output, aortic developed pressure and cardiac power were preserved in ADQ-/- mice. Rates of fatty acid oxidation, glucose oxidation and glycolysis were unchanged between groups. While myocardial oxygen consumption was slightly reduced (-24%) in ADQ-/- mice in isolated working hearts, rates of maximal ADP-stimulated mitochondrial oxygen consumption and ATP synthesis in saponin-permeabilized cardiac fibers were preserved in ADQ-/- mice with glutamate, pyruvate or palmitoyl-carnitine as a substrate. In addition, enzymatic activity of respiratory complexes I and II was unchanged between groups. Phosphorylation of AMP-activated protein kinase and SIRT1 activity were not decreased, expression and acetylation of PGC-1α were unchanged, and mitochondrial content of OXPHOS subunits was not decreased in ADQ-/- mice. Finally, increasing energy demands due to prolonged subcutaneous infusion of isoproterenol did not differentially affect cardiac contractility or mitochondrial function in ADQ-/- mice compared to WT. Thus, mitochondrial and contractile function are preserved in hearts of mice lacking adiponectin, suggesting that adiponectin may be expendable in the regulation of mitochondrial energetics and contractile function in the heart under non-pathological conditions.
Conflict of interest statement
Figures
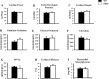
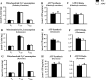



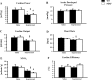

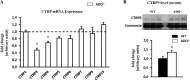
Similar articles
-
SIRT3 deficiency impairs mitochondrial and contractile function in the heart.Basic Res Cardiol. 2015;110(4):36. doi: 10.1007/s00395-015-0493-6. Epub 2015 May 12. Basic Res Cardiol. 2015. PMID: 25962702
-
The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis.Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H185-96. doi: 10.1152/ajpheart.00081.2008. Epub 2008 May 16. Am J Physiol Heart Circ Physiol. 2008. PMID: 18487436 Free PMC article.
-
PGC-1β deficiency accelerates the transition to heart failure in pressure overload hypertrophy.Circ Res. 2011 Sep 16;109(7):783-93. doi: 10.1161/CIRCRESAHA.111.243964. Epub 2011 Jul 28. Circ Res. 2011. PMID: 21799152 Free PMC article.
-
Heart failure: is there an energy deficit contributing to contractile dysfunction?Basic Res Cardiol. 1998 Feb;93(1):1-10. doi: 10.1007/s003950050055. Basic Res Cardiol. 1998. PMID: 9538931 Review.
-
The 'Goldilocks zone' of fatty acid metabolism; to ensure that the relationship with cardiac function is just right.Clin Sci (Lond). 2017 Jul 24;131(16):2079-2094. doi: 10.1042/CS20160671. Print 2017 Aug 15. Clin Sci (Lond). 2017. PMID: 28739841 Review.
Cited by
-
Adiponectin protects against myocardial ischemia-reperfusion injury: a systematic review and meta-analysis of preclinical animal studies.Lipids Health Dis. 2024 Feb 17;23(1):51. doi: 10.1186/s12944-024-02028-w. Lipids Health Dis. 2024. PMID: 38368320 Free PMC article.
-
Adipokines and Inflammation: Focus on Cardiovascular Diseases.Int J Mol Sci. 2020 Oct 18;21(20):7711. doi: 10.3390/ijms21207711. Int J Mol Sci. 2020. PMID: 33081064 Free PMC article. Review.
-
HDAC inhibition induces autophagy and mitochondrial biogenesis to maintain mitochondrial homeostasis during cardiac ischemia/reperfusion injury.J Mol Cell Cardiol. 2019 May;130:36-48. doi: 10.1016/j.yjmcc.2019.03.008. Epub 2019 Mar 14. J Mol Cell Cardiol. 2019. PMID: 30880250 Free PMC article.
-
Low Cytochrome Oxidase 1 Links Mitochondrial Dysfunction to Atherosclerosis in Mice and Pigs.PLoS One. 2017 Jan 25;12(1):e0170307. doi: 10.1371/journal.pone.0170307. eCollection 2017. PLoS One. 2017. PMID: 28122051 Free PMC article.
-
Adiponectin Protects Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury by Suppressing Autophagy.J Immunol Res. 2022 Oct 17;2022:8433464. doi: 10.1155/2022/8433464. eCollection 2022. J Immunol Res. 2022. PMID: 36300016 Free PMC article.
References
-
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221: 286–289. - PubMed
-
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423: 762–769. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

