A Plasmodium phospholipase is involved in disruption of the liver stage parasitophorous vacuole membrane
- PMID: 25786000
- PMCID: PMC4364735
- DOI: 10.1371/journal.ppat.1004760
A Plasmodium phospholipase is involved in disruption of the liver stage parasitophorous vacuole membrane
Abstract
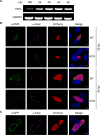
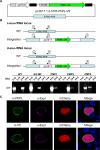
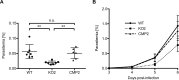
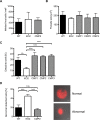
The coordinated exit of intracellular pathogens from host cells is a process critical to the success and spread of an infection. While phospholipases have been shown to play important roles in bacteria host cell egress and virulence, their role in the release of intracellular eukaryotic parasites is largely unknown. We examined a malaria parasite protein with phospholipase activity and found it to be involved in hepatocyte egress. In hepatocytes, Plasmodium parasites are surrounded by a parasitophorous vacuole membrane (PVM), which must be disrupted before parasites are released into the blood. However, on a molecular basis, little is known about how the PVM is ruptured. We show that Plasmodium berghei phospholipase, PbPL, localizes to the PVM in infected hepatocytes. We provide evidence that parasites lacking PbPL undergo completely normal liver stage development until merozoites are produced but have a defect in egress from host hepatocytes. To investigate this further, we established a live-cell imaging-based assay, which enabled us to study the temporal dynamics of PVM rupture on a quantitative basis. Using this assay we could show that PbPL-deficient parasites exhibit impaired PVM rupture, resulting in delayed parasite egress. A wild-type phenotype could be re-established by gene complementation, demonstrating the specificity of the PbPL deletion phenotype. In conclusion, we have identified for the first time a Plasmodium phospholipase that is important for PVM rupture and in turn for parasite exit from the infected hepatocyte and therefore established a key role of a parasite phospholipase in egress.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
A key role for Plasmodium subtilisin-like SUB1 protease in egress of malaria parasites from host hepatocytes.J Biol Chem. 2013 Nov 15;288(46):33336-46. doi: 10.1074/jbc.M113.513234. Epub 2013 Oct 2. J Biol Chem. 2013. PMID: 24089525 Free PMC article.
-
Disrupting a Plasmodium berghei putative phospholipase impairs efficient egress of merosomes.Int J Parasitol. 2022 Jul;52(8):547-558. doi: 10.1016/j.ijpara.2022.03.002. Epub 2022 Apr 12. Int J Parasitol. 2022. PMID: 35427579
-
Long-term live imaging reveals cytosolic immune responses of host hepatocytes against Plasmodium infection and parasite escape mechanisms.Autophagy. 2015;11(9):1561-79. doi: 10.1080/15548627.2015.1067361. Autophagy. 2015. PMID: 26208778 Free PMC article.
-
Moving on: How malaria parasites exit the liver.Mol Microbiol. 2024 Mar;121(3):328-340. doi: 10.1111/mmi.15141. Epub 2023 Aug 21. Mol Microbiol. 2024. PMID: 37602900 Review.
-
Molecular mechanisms of host cell egress by malaria parasites.Int J Med Microbiol. 2012 Oct;302(4-5):172-8. doi: 10.1016/j.ijmm.2012.07.003. Epub 2012 Aug 27. Int J Med Microbiol. 2012. PMID: 22951233 Review.
Cited by
-
Overlapping and distinct roles of CDPK family members in the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium berghei.PLoS Pathog. 2020 Aug 31;16(8):e1008131. doi: 10.1371/journal.ppat.1008131. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866196 Free PMC article.
-
Patatin-like phospholipases in microbial infections with emerging roles in fatty acid metabolism and immune regulation by Apicomplexa.Mol Microbiol. 2018 Jan;107(1):34-46. doi: 10.1111/mmi.13871. Epub 2017 Nov 23. Mol Microbiol. 2018. PMID: 29090840 Free PMC article. Review.
-
Genome-Scale Identification of Essential Metabolic Processes for Targeting the Plasmodium Liver Stage.Cell. 2019 Nov 14;179(5):1112-1128.e26. doi: 10.1016/j.cell.2019.10.030. Cell. 2019. PMID: 31730853 Free PMC article.
-
A Novel and Conserved Plasmodium Sporozoite Membrane Protein SPELD is Required for Maturation of Exo-erythrocytic Forms.Sci Rep. 2017 Jan 9;7:40407. doi: 10.1038/srep40407. Sci Rep. 2017. PMID: 28067322 Free PMC article.
-
Structural Features of Apicomplexan Pore-Forming Proteins and Their Roles in Parasite Cell Traversal and Egress.Toxins (Basel). 2017 Aug 29;9(9):265. doi: 10.3390/toxins9090265. Toxins (Basel). 2017. PMID: 28850082 Free PMC article. Review.
References
-
- Ward GE, Miller LH, Dvorak JA (1993) The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J Cell Sci 106 (Pt 1): 237–248. - PubMed
-
- Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, et al. (2006) Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313: 1287–1290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

