Mitochondria in the regulation of innate and adaptive immunity
- PMID: 25786173
- PMCID: PMC4365295
- DOI: 10.1016/j.immuni.2015.02.002
Mitochondria in the regulation of innate and adaptive immunity
Abstract
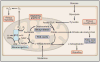
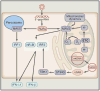
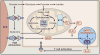
Mitochondria are well appreciated for their role as biosynthetic and bioenergetic organelles. In the past two decades, mitochondria have emerged as signaling organelles that contribute critical decisions about cell proliferation, death, and differentiation. Mitochondria not only sustain immune cell phenotypes but also are necessary for establishing immune cell phenotype and their function. Mitochondria can rapidly switch from primarily being catabolic organelles generating ATP to anabolic organelles that generate both ATP and building blocks for macromolecule synthesis. This enables them to fulfill appropriate metabolic demands of different immune cells. Mitochondria have multiple mechanisms that allow them to activate signaling pathways in the cytosol including altering in AMP/ATP ratio, the release of ROS and TCA cycle metabolites, as well as the localization of immune regulatory proteins on the outer mitochondrial membrane. In this Review, we discuss the evidence and mechanisms that mitochondrial dependent signaling controls innate and adaptive immune responses.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2014;35:88–93. - PubMed
-
- Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med. 2014;20:1327–1333. - PubMed
-
- Camões F, Bonekamp NA, Delille HK, Schrader M. Organelle dynamics and dysfunction: A closer link between peroxisomes and mitochondria. J Inherit Metab Dis. 2009;32:163–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

