Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress
- PMID: 25786204
- PMCID: PMC4461587
- DOI: 10.2119/molmed.2014.00158
Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress
Abstract
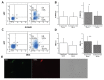
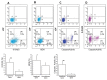
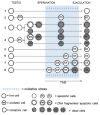
Sperm DNA fragmentation (sDF) represents a threat to male fertility, human reproduction and the health of the offspring. The causes of sDF are still unclear, even if apoptosis, oxidative assault and defects in chromatin maturation are hypothesized. Using multicolor flow cytometry and sperm sorting, we challenged the three hypothesized mechanisms by simultaneously evaluating sDF and signs of oxidative damage (8-hydroxy, 2'-deoxyguanosine [8-OHdG] and malondialdehyde [MDA]), apoptosis (caspase activity and cleaved poly[ADP-ribose] polymerase [cPARP]) and sperm immaturity (creatine phosphokinase [CK] and excess of residual histones). Active caspases and c-PARP were concomitant with sDF in a high percentage of spermatozoa (82.6% ± 9.1% and 53.5% ± 16.4%, respectively). Excess of residual histones was significantly higher in DNA-fragmented sperm versus sperm without DNA fragmentation (74.8% ± 17.5% and 37.3% ± 16.6%, respectively, p < 0.005), and largely concomitant with active caspases. Conversely, oxidative damage was scarcely concomitant with sDF in the total sperm population, at variance with live sperm, where 8-OHdG and MDA were clearly associated to sDF. In addition, most live cells with active caspase also showed 8-OHdG, suggesting activation of apoptotic pathways in oxidative-injured live cells. This is the first investigation on the origin of sDF directly evaluating the simultaneous presence of the signs of the hypothesized mechanisms with DNA breaks at the single cell level. The results indicate that the main pathway leading to sperm DNA breaks is a process of apoptosis, likely triggered by an impairment of chromatin maturation in the testis and by oxidative stress during the transit in the male genital tract. These findings are highly relevant for clinical studies on the effects of drugs on sDF and oxidative stress in infertile men and for the development of new therapeutic strategies.
Figures





References
-
- Ahmadi A, Ng SC. Developmental capacity of damaged spermatozoa. Hum Reprod. 1999;14:2279–85. - PubMed
-
- Singh NP, et al. Abundant alkali-sensitive sites in DNA of human and mouse sperm. Exp Cell Res. 1989;184:461–70. - PubMed
-
- Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells. Exp Cell Res. 1993;207:202–5. - PubMed
-
- Morris ID. Sperm DNA damage and cancer treatment. Int J Androl. 2002;25:255–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

