Gold carbene or carbenoid: is there a difference?
- PMID: 25786384
- PMCID: PMC4648030
- DOI: 10.1002/chem.201406318
Gold carbene or carbenoid: is there a difference?
Abstract
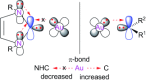
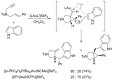
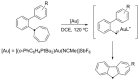
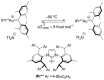
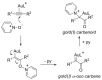
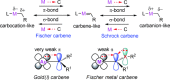
By reviewing the recent progress on the elucidation of the structure of gold carbenes and the definitions of metal carbenes and carbenoids, we recommend to use the term gold carbene to describe gold carbene-like intermediates, regardless of whether the carbene or carbocation extreme resonance dominates. Gold carbenes, because of the weak metal-to-carbene π-back-donation and their strongly electrophilic reactivity, could be classified into the broader family of Fischer carbenes, although their behavior and properties are very specific.
Keywords: Fischer carbenes; carbenes; carbenoids; carbocations; gold.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






References
-
- Gorin DJ, Toste FD. Nature. 2007;446:395–403. For recent reviews on homogeneous gold catalysis, see. - PubMed
-
- Jiménez-Núñez E, Echavarren AM. Chem. Rev. 2008;108:3326–3350. - PubMed
-
- Díaz-Requejo MM, Pérez PJ. Chem. Rev. 2008;108:3379–3394. - PubMed
-
- Fürstner A, Davies PW. Angew. Chem. Int. Ed. 2007;46:3410–3449. - PubMed
-
- Angew. Chem. 2007;119
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

