Correlation of two-photon in vivo imaging and FIB/SEM microscopy
- PMID: 25786682
- PMCID: PMC4672704
- DOI: 10.1111/jmi.12231
Correlation of two-photon in vivo imaging and FIB/SEM microscopy
Abstract
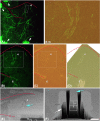
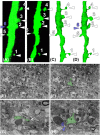
Advances in the understanding of brain functions are closely linked to the technical developments in microscopy. In this study, we describe a correlative microscopy technique that offers a possibility of combining two-photon in vivo imaging with focus ion beam/scanning electron microscope (FIB/SEM) techniques. Long-term two-photon in vivo imaging allows the visualization of functional interactions within the brain of a living organism over the time, and therefore, is emerging as a new tool for studying the dynamics of neurodegenerative diseases, such as Alzheimer's disease. However, light microscopy has important limitations in revealing alterations occurring at the synaptic level and when this is required, electron microscopy is mandatory. FIB/SEM microscopy is a novel tool for three-dimensional high-resolution reconstructions, since it acquires automated serial images at ultrastructural level. Using FIB/SEM imaging, we observed, at 10 nm isotropic resolution, the same dendrites that were imaged in vivo over 9 days. Thus, we analyzed their ultrastructure and monitored the dynamics of the neuropil around them. We found that stable spines (present during the 9 days of imaging) formed typical asymmetric contacts with axons, whereas transient spines (present only during one day of imaging) did not form a synaptic contact. Our data suggest that the morphological classification that was assigned to a dendritic spine according to the in vivo images did not fit with its ultrastructural morphology. The correlative technique described herein is likely to open opportunities for unravelling the earlier unrecognized complexity of the nervous system.
Keywords: Dendritic spine; electron microscopy; green fluorescent protein; three-dimensional reconstruction.
© 2015 The Authors Journal of Microscopy © 2015 Royal Microscopical Society.
Figures

References
-
- Bishop D, Nikic I, Brinkoetter M, Knecht S, Potz S, Kerschensteiner M. Misgeld T. Near-infrared branding efficiently correlates light and electron microscopy. Nat. Methods. 2011;8:568–570. - PubMed
-
- Blazquez-Llorca L, Merchan-Perez A, Rodriguez JR, Gascon J. DeFelipe J. FIB/SEM technology and Alzheimer’s disease: three-dimensional analysis of human cortical synapses. J. Alzheimers Dis. 2013;34:995–1013. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

