The aggregation-prone intracellular serpin SRP-2 fails to transit the ER in Caenorhabditis elegans
- PMID: 25786854
- PMCID: PMC4423363
- DOI: 10.1534/genetics.115.176180
The aggregation-prone intracellular serpin SRP-2 fails to transit the ER in Caenorhabditis elegans
Abstract
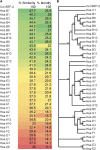
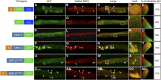
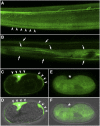
Familial encephalopathy with neuroserpin inclusions bodies (FENIB) is a serpinopathy that induces a rare form of presenile dementia. Neuroserpin contains a classical signal peptide and like all extracellular serine proteinase inhibitors (serpins) is secreted via the endoplasmic reticulum (ER)-Golgi pathway. The disease phenotype is due to gain-of-function missense mutations that cause neuroserpin to misfold and aggregate within the ER. In a previous study, nematodes expressing a homologous mutation in the endogenous Caenorhabditis elegans serpin, srp-2, were reported to model the ER proteotoxicity induced by an allele of mutant neuroserpin. Our results suggest that SRP-2 lacks a classical N-terminal signal peptide and is a member of the intracellular serpin family. Using confocal imaging and an ER colocalization marker, we confirmed that GFP-tagged wild-type SRP-2 localized to the cytosol and not the ER. Similarly, the aggregation-prone SRP-2 mutant formed intracellular inclusions that localized to the cytosol. Interestingly, wild-type SRP-2, targeted to the ER by fusion to a cleavable N-terminal signal peptide, failed to be secreted and accumulated within the ER lumen. This ER retention phenotype is typical of other obligate intracellular serpins forced to translocate across the ER membrane. Neuroserpin is a secreted protein that inhibits trypsin-like proteinase. SRP-2 is a cytosolic serpin that inhibits lysosomal cysteine peptidases. We concluded that SRP-2 is neither an ortholog nor a functional homolog of neuroserpin. Furthermore, animals expressing an aggregation-prone mutation in SRP-2 do not model the ER proteotoxicity associated with FENIB.
Keywords: ER stress; SRP-2; familial encephalopathy with neuroserpin inclusions; neuroserpin; proteostasis.
Copyright © 2015 by the Genetics Society of America.
Figures
References
-
- Barlow A. L., Macleod A., Noppen S., Sanderson J., Guerin C. J., 2010. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of Pearson’s correlation coefficient. Microsc. Microanal. 16: 710–724. - PubMed
-
- Belin D., 1993. Biology and facultative secretion of plasminogen activator inhibitor-2. Thromb. Haemost. 70: 144–147. - PubMed
-
- Belin D., Guzman L. M., Bost S., Konakova M., Silva F., et al. , 2004. Functional activity of eukaryotic signal sequences in Escherichia coli: the ovalbumin family of serine protease inhibitors. J. Mol. Biol. 335: 437–453. - PubMed
-
- Benyair R., Ogen-Shtern N., Lederkremer G. Z., 2014. Glycan regulation of ER-associated degradation through compartmentalization. Semin. Cell Dev. Biol. (in press). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

