Acetylation stimulates the epithelial sodium channel by reducing its ubiquitination and degradation
- PMID: 25787079
- PMCID: PMC4432271
- DOI: 10.1074/jbc.M114.635540
Acetylation stimulates the epithelial sodium channel by reducing its ubiquitination and degradation
Abstract
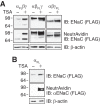
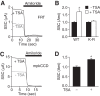
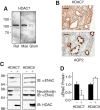
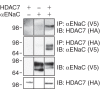
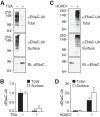
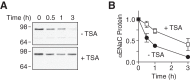
The epithelial Na(+) channel (ENaC) functions as a pathway for Na(+) absorption in the kidney and lung, where it is crucial for Na(+) homeostasis and blood pressure regulation. ENaC is regulated in part through signaling pathways that control the ubiquitination state of ENaC lysines. A defect in ubiquitination causes Liddle syndrome, an inherited form of hypertension. Here we determined that α-, β-, and γENaC are also substrates for lysine acetylation. Trichostatin A (TSA), a histone deacetylase inhibitor, enhanced ENaC acetylation and increased ENaC abundance in the total cell lysate and at the cell surface. Moreover, TSA increased ENaC current in Fischer rat thyroid and kidney collecting duct epithelia. We found that HDAC7 is expressed in the kidney collecting duct, supporting a potential role for this histone deacetylase in ENaC regulation. HDAC7 overexpression reduced ENaC abundance and ENaC current, whereas ENaC abundance and current were increased by silencing of HDAC7. ENaC and HDAC7 form a complex, as detected by coimmunoprecipitation. We observed a reciprocal relationship between acetylation and ubiquitination; TSA reduced ENaC ubiquitination, whereas HDAC7 increased ubiquitination. By reducing ENaC ubiquitination, TSA decreased the rate of ENaC degradation. Thus, acetylation increases epithelial Na(+) absorption by antagonizing ENaC ubiquitination. This stabilizes ENaC, and hence, increases its abundance at the cell surface.
Keywords: Amiloride; Epithelia; Epithelial Sodium Channel (ENaC); Histone Deacetylase (HDAC); Hypertension; Lysine Acetylation; Protein Degradation; Ubiquitylation (Ubiquitination).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Trichostatin A blocks aldosterone-induced Na+ transport and control of serum- and glucocorticoid-inducible kinase 1 in cortical collecting duct cells.Br J Pharmacol. 2019 Dec;176(24):4708-4719. doi: 10.1111/bph.14837. Epub 2019 Oct 25. Br J Pharmacol. 2019. PMID: 31423568 Free PMC article.
-
Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC.J Biol Chem. 2007 Jul 13;282(28):20207-12. doi: 10.1074/jbc.M611329200. Epub 2007 May 14. J Biol Chem. 2007. PMID: 17502380
-
Ubiquitin-specific peptidase 8 (USP8) regulates endosomal trafficking of the epithelial Na+ channel.J Biol Chem. 2013 Feb 22;288(8):5389-97. doi: 10.1074/jbc.M112.425272. Epub 2013 Jan 7. J Biol Chem. 2013. PMID: 23297398 Free PMC article.
-
Regulation of sodium transport by ENaC in the kidney.Curr Opin Nephrol Hypertens. 2010 Jan;19(1):98-105. doi: 10.1097/MNH.0b013e328332bda4. Curr Opin Nephrol Hypertens. 2010. PMID: 19996890 Free PMC article. Review.
-
The epithelial sodium channel and the control of sodium balance.Biochim Biophys Acta. 2010 Dec;1802(12):1159-65. doi: 10.1016/j.bbadis.2010.06.014. Epub 2010 Jun 27. Biochim Biophys Acta. 2010. PMID: 20600867 Review.
Cited by
-
CBP mediated DOT1L acetylation confers DOT1L stability and promotes cancer metastasis.Theranostics. 2020 Jan 1;10(4):1758-1776. doi: 10.7150/thno.39013. eCollection 2020. Theranostics. 2020. PMID: 32042335 Free PMC article.
-
BDH1 acetylation at K116 modulates milk fat production in dairy goats.Res Sq [Preprint]. 2025 Jul 29:rs.3.rs-7156146. doi: 10.21203/rs.3.rs-7156146/v1. Res Sq. 2025. PMID: 40766244 Free PMC article. Preprint.
-
Trichostatin A blocks aldosterone-induced Na+ transport and control of serum- and glucocorticoid-inducible kinase 1 in cortical collecting duct cells.Br J Pharmacol. 2019 Dec;176(24):4708-4719. doi: 10.1111/bph.14837. Epub 2019 Oct 25. Br J Pharmacol. 2019. PMID: 31423568 Free PMC article.
-
BK channel deacetylation by SIRT1 in dentate gyrus regulates anxiety and response to stress.Commun Biol. 2018 Jun 28;1:82. doi: 10.1038/s42003-018-0088-5. eCollection 2018. Commun Biol. 2018. PMID: 30271963 Free PMC article.
-
The cardioprotective role of sirtuins is mediated in part by regulating KATP channel surface expression.Am J Physiol Cell Physiol. 2023 May 1;324(5):C1017-C1027. doi: 10.1152/ajpcell.00459.2022. Epub 2023 Mar 6. Am J Physiol Cell Physiol. 2023. PMID: 36878847 Free PMC article.
References
-
- Schild L. (2004) The epithelial sodium channel: from molecule to disease. Rev. Physiol. Biochem. Pharmacol. 151, 93–107 - PubMed
-
- Snyder P. M. (2005) Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology 146, 5079–5085 - PubMed
-
- Lifton R. P. (1996) Molecular genetics of human blood pressure variation. Science 272, 676–680 - PubMed
-
- Snyder P. M., Price M. P., McDonald F. J., Adams C. M., Volk K. A., Zeiher B. G., Stokes J. B., Welsh M. J. (1995) Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+ channel. Cell 83, 969–978 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

