Zinc inhibits Hedgehog autoprocessing: linking zinc deficiency with Hedgehog activation
- PMID: 25787080
- PMCID: PMC4416862
- DOI: 10.1074/jbc.M114.623264
Zinc inhibits Hedgehog autoprocessing: linking zinc deficiency with Hedgehog activation
Abstract
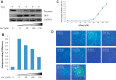
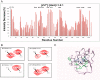
Zinc is an essential trace element with wide-ranging biological functions, whereas the Hedgehog (Hh) signaling pathway plays crucial roles in both development and disease. Here we show that there is a mechanistic link between zinc and Hh signaling. The upstream activator of Hh signaling, the Hh ligand, originates from Hh autoprocessing, which converts the Hh precursor protein to the Hh ligand. In an in vitro Hh autoprocessing assay we show that zinc inhibits Hh autoprocessing with a Ki of 2 μm. We then demonstrate that zinc inhibits Hh autoprocessing in a cellular environment with experiments in primary rat astrocyte culture. Solution NMR reveals that zinc binds the active site residues of the Hh autoprocessing domain to inhibit autoprocessing, and isothermal titration calorimetry provided the thermodynamics of the binding. In normal physiology, zinc likely acts as a negative regulator of Hh autoprocessing and inhibits the generation of Hh ligand and Hh signaling. In many diseases, zinc deficiency and elevated level of Hh ligand co-exist, including prostate cancer, lung cancer, ovarian cancer, and autism. Our data suggest a causal relationship between zinc deficiency and the overproduction of Hh ligand.
Keywords: Cancer; Hedgehog Autoprocessing; Hedgehog Signaling Pathway; Isothermal Titration Calorimetry (ITC); Nuclear Magnetic Resonance (NMR); Zinc.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Briscoe J., Thérond P. P. (2013) The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14, 416–429 - PubMed
-
- Karhadkar S. S., Bova G. S., Abdallah N., Dhara S., Gardner D., Maitra A., Isaacs J. T., Berman D. M., Beachy P. A. (2004) Hedgehog signalling in prostate regeneration, neoplasia, and metastasis. Nature 431, 707–712 - PubMed
-
- Watkins D. N., Berman D. M., Burkholder S. G., Wang B., Beachy P. A., Baylin S. B. (2003) Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422, 313–317 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

