Regional gray matter atrophy in relapsing remitting multiple sclerosis: baseline analysis of multi-center data
- PMID: 25787188
- PMCID: PMC4366621
- DOI: 10.1016/j.msard.2015.01.004
Regional gray matter atrophy in relapsing remitting multiple sclerosis: baseline analysis of multi-center data
Abstract
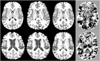
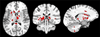
Regional gray matter (GM) atrophy in multiple sclerosis (MS) at disease onset and its temporal variation can provide objective information regarding disease evolution. An automated pipeline for estimating atrophy of various GM structures was developed using tensor based morphometry (TBM) and implemented on a multi-center sub-cohort of 1008 relapsing remitting MS (RRMS) patients enrolled in a Phase 3 clinical trial. Four hundred age and gender matched healthy controls were used for comparison. Using the analysis of covariance, atrophy differences between MS patients and healthy controls were assessed on a voxel-by-voxel analysis. Regional GM atrophy was observed in a number of deep GM structures that included thalamus, caudate nucleus, putamen, and cortical GM regions. General linear regression analysis was performed to analyze the effects of age, gender, and scanner field strength, and imaging sequence on the regional atrophy. Correlations between regional GM volumes and expanded disability status scale (EDSS) scores, disease duration (DD), T2 lesion load (T2 LL), T1 lesion load (T1 LL), and normalized cerebrospinal fluid (nCSF) were analyzed using Pearson׳s correlation coefficient. Thalamic atrophy observed in MS patients compared to healthy controls remained consistent within subgroups based on gender and scanner field strength. Weak correlations between thalamic volume and EDSS (r=-0.133; p<0.001) and DD (r=-0.098; p=0.003) were observed. Of all the structures, thalamic volume moderately correlated with T2 LL (r=-0.492; P-value<0.001), T1 LL (r=-0.473; P-value<0.001) and nCSF (r=-0.367; P-value<0.001).
Keywords: CombiRx; Regional atrophy; Relapsing remitting multiple sclerosis; Tensor based morphometry; Unbiased template.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures




References
-
- Aubert-Broche B, Fonov V, Ghassemi R, Narayanan S, Arnold DL, Banwell B, Sled JG, Collins DL. Regional brain atrophy in children with multiple sclerosis. Neuroimage. 2011;58:409–415. - PubMed
-
- Audoin B, Zaaraoui W, Reuter F, Rico A, Malikova I, Confort-Gouny S, Cozzone PJ, Pelletier J, Ranjeva JP. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:690–695. - PubMed
-
- Ashburner J. A Fast Diffeomorphic Image Registration Algorithm. NeuroImage. 2007;38:95–113. - PubMed
-
- Bakshi R, Benedict RH, Bermel RA, Jacobs L. Regional brain atrophy is associated with physical disability in multiple sclerosis: semiquantitative magnetic resonance imaging and relationship to clinical findings. J Neuroimaging. 2001;11:129–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

