Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti
- PMID: 25787763
- PMCID: PMC4390297
- DOI: 10.1126/scitranslmed.3010370
Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti
Abstract
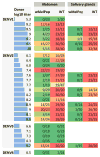
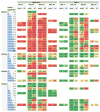
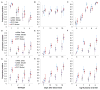
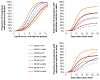
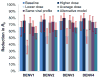
Dengue is the most common arboviral infection of humans and is a public health burden in more than 100 countries. Aedes aegypti mosquitoes stably infected with strains of the intracellular bacterium Wolbachia are resistant to dengue virus (DENV) infection and are being tested in field trials. To mimic field conditions, we experimentally assessed the vector competence of A. aegypti carrying the Wolbachia strains wMel and wMelPop after challenge with viremic blood from dengue patients. We found that wMelPop conferred strong resistance to DENV infection of mosquito abdomen tissue and largely prevented disseminated infection. wMel conferred less resistance to infection of mosquito abdomen tissue, but it did reduce the prevalence of mosquitoes with infectious saliva. A mathematical model of DENV transmission incorporating the dynamics of viral infection in humans and mosquitoes was fitted to the data collected. Model predictions suggested that wMel would reduce the basic reproduction number, R0, of DENV transmission by 66 to 75%. Our results suggest that establishment of wMelPop-infected A. aegypti at a high frequency in a dengue-endemic setting would result in the complete abatement of DENV transmission. Establishment of wMel-infected A. aegypti is also predicted to have a substantial effect on transmission that would be sufficient to eliminate dengue in low or moderate transmission settings but may be insufficient to achieve complete control in settings where R0 is high. These findings develop a framework for selecting Wolbachia strains for field releases and for calculating their likely impact.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

References
-
- Simmons CP, Farrar JJ, Nguyen V, Wills vB. Dengue. The New England journal of medicine. 2012;366:1423–1432. - PubMed
-
- McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, O’Neill SL. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. - PubMed
-
- Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O’Neill SL, Hoffmann AA. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. - PubMed
-
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, Hugo LE, Johnson KN, Kay BH, McGraw EA, van den Hurk AF, Ryan PA, O’Neill SL. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

