Spatial pattern of spontaneous retinal waves instructs retinotopic map refinement more than activity frequency
- PMID: 25787992
- PMCID: PMC4697738
- DOI: 10.1002/dneu.22288
Spatial pattern of spontaneous retinal waves instructs retinotopic map refinement more than activity frequency
Abstract
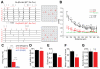
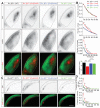
Spontaneous activity during early development is necessary for the formation of precise neural connections, but it remains uncertain whether activity plays an instructive or permissive role in brain wiring. In the visual system, retinal ganglion cell (RGC) projections to the brain form two prominent sensory maps, one reflecting eye of origin and the other retinotopic location. Recent studies provide compelling evidence supporting an instructive role for spontaneous retinal activity in the development of eye-specific projections, but evidence for a similarly instructive role in the development of retinotopy is more equivocal. Here, we report on experiments in which we knocked down the expression of β2-containing nicotinic acetylcholine receptors (β2-nAChRs) specifically in the retina through a Cre-loxP recombination strategy. Overall levels of spontaneous retinal activity in retina-specific β2-nAChR mutant mice (Rx-β2cKO), examined in vitro and in vivo, were reduced to a degree comparable to that observed in whole animal β2-nAChR mouse mutants (β2KO). However, many residual spontaneous waves in Rx-β2cKO mice displayed local propagating features with strong correlations between nearby but not distant RGCs typical of waves observed in wild-type (WT) but not β2KO mice. We further observed that eye-specific segregation was disrupted in Rx-β2cKO mice, but retinotopy was spared in a competition-dependent manner. These results suggest that propagating patterns of spontaneous retinal waves are essential for normal development of the retinotopic map, even while overall activity levels are significantly reduced, and support an instructive role for spontaneous retinal activity in both eye-specific segregation and retinotopic refinement.
Keywords: activity-dependent; retinotopy; vision.
© 2015 Wiley Periodicals, Inc.
Figures






References
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J. Neurosci. 2000;20:7672–81. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

