The effect of dose on the antimalarial efficacy of artemether-lumefantrine: a systematic review and pooled analysis of individual patient data
- PMID: 25788162
- PMCID: PMC4632191
- DOI: 10.1016/S1473-3099(15)70024-1
The effect of dose on the antimalarial efficacy of artemether-lumefantrine: a systematic review and pooled analysis of individual patient data
Erratum in
-
Corrections.Lancet Infect Dis. 2015 May;15(5):505. doi: 10.1016/S1473-3099(14)71016-3. Epub 2015 Feb 11. Lancet Infect Dis. 2015. PMID: 25932575
Abstract
Background: Artemether-lumefantrine is the most widely used artemisinin-based combination therapy for malaria, although treatment failures occur in some regions. We investigated the effect of dosing strategy on efficacy in a pooled analysis from trials done in a wide range of malaria-endemic settings.
Methods: We searched PubMed for clinical trials that enrolled and treated patients with artemether-lumefantrine and were published from 1960 to December, 2012. We merged individual patient data from these trials by use of standardised methods. The primary endpoint was the PCR-adjusted risk of Plasmodium falciparum recrudescence by day 28. Secondary endpoints consisted of the PCR-adjusted risk of P falciparum recurrence by day 42, PCR-unadjusted risk of P falciparum recurrence by day 42, early parasite clearance, and gametocyte carriage. Risk factors for PCR-adjusted recrudescence were identified using Cox's regression model with frailty shared across the study sites.
Findings: We included 61 studies done between January, 1998, and December, 2012, and included 14,327 patients in our analyses. The PCR-adjusted therapeutic efficacy was 97·6% (95% CI 97·4-97·9) at day 28 and 96·0% (95·6-96·5) at day 42. After controlling for age and parasitaemia, patients prescribed a higher dose of artemether had a lower risk of having parasitaemia on day 1 (adjusted odds ratio [OR] 0·92, 95% CI 0·86-0·99 for every 1 mg/kg increase in daily artemether dose; p=0·024), but not on day 2 (p=0·69) or day 3 (0·087). In Asia, children weighing 10-15 kg who received a total lumefantrine dose less than 60 mg/kg had the lowest PCR-adjusted efficacy (91·7%, 95% CI 86·5-96·9). In Africa, the risk of treatment failure was greatest in malnourished children aged 1-3 years (PCR-adjusted efficacy 94·3%, 95% CI 92·3-96·3). A higher artemether dose was associated with a lower gametocyte presence within 14 days of treatment (adjusted OR 0·92, 95% CI 0·85-0·99; p=0·037 for every 1 mg/kg increase in total artemether dose).
Interpretation: The recommended dose of artemether-lumefantrine provides reliable efficacy in most patients with uncomplicated malaria. However, therapeutic efficacy was lowest in young children from Asia and young underweight children from Africa; a higher dose regimen should be assessed in these groups.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
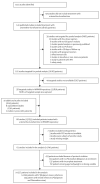
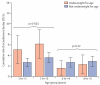
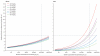
Comment in
-
A localised threat to an excellent antimalarial drug.Lancet Infect Dis. 2015 Jun;15(6):623-4. doi: 10.1016/S1473-3099(15)70100-3. Epub 2015 Mar 16. Lancet Infect Dis. 2015. PMID: 25788161 No abstract available.
References
-
- WHO [accessed March 19, 2014];World malaria report 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/en/
-
- Jiao X, Liu GY, Shan CO, et al. Phase II trial in China of a new, rapidly-acting and effective oral antimalarial, CGP 56697, for the treatment of Plasmodium falciparum malaria. Southeast Asian J Trop Med Public Health. 1997;28:476–81. - PubMed
-
- Hatz C, Abdulla S, Mull R, et al. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1–5 years. Trop Med Int Health. 1998;3:498–504. - PubMed

