Porous three-dimensional carbon nanotube scaffolds for tissue engineering
- PMID: 25788440
- PMCID: PMC4552611
- DOI: 10.1002/jbm.a.35449
Porous three-dimensional carbon nanotube scaffolds for tissue engineering
Abstract
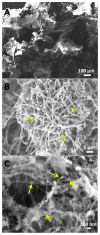
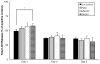
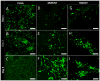
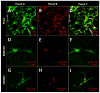
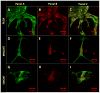
Assembly of carbon nanomaterials into three-dimensional (3D) architectures is necessary to harness their unique physiochemical properties for tissue engineering and regenerative medicine applications. Herein, we report the fabrication and comprehensive cytocompatibility assessment of 3D chemically crosslinked macrosized (5-8 mm height and 4-6 mm diameter) porous carbon nanotube (CNT) scaffolds. Scaffolds prepared via radical initiated thermal crosslinking of single- or multiwalled CNTs (SWCNTs and MWCNTs) possess high porosity (>80%), and nano-, micro-, and macroscale interconnected pores. MC3T3 preosteoblast cells on MWCNT and SWCNT scaffolds showed good cell viability comparable to poly(lactic-co-glycolic) acid (PLGA) scaffolds after 5 days. Confocal live cell and immunofluorescence imaging showed that MC3T3 cells were metabolically active and could attach, proliferate, and infiltrate MWCNT and SWCNT scaffolds. SEM imaging corroborated cell attachment and spreading and suggested that cell morphology is governed by scaffold surface roughness. MC3T3 cells were elongated on scaffolds with high surface roughness (MWCNTs) and rounded on scaffolds with low surface roughness (SWCNTs). The surface roughness of scaffolds may be exploited to control cellular morphology and, in turn, govern cell fate. These results indicate that crosslinked MWCNTs and SWCNTs scaffolds are cytocompatible, and open avenues toward development of multifunctional all-carbon scaffolds for tissue engineering applications.
Keywords: carbon nanotubes; cytotoxicity; scaffolds; three dimensional; tissue engineering.
© 2015 Wiley Periodicals, Inc.
Figures




References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. - PubMed
-
- Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nature materials. 2014;13:558–69. - PubMed
-
- Peltola SM, Melchels FP, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Annals of medicine. 2008;40:268–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

