A Phase I/II Trial of BNC105P with Everolimus in Metastatic Renal Cell Carcinoma
- PMID: 25788492
- PMCID: PMC4526387
- DOI: 10.1158/1078-0432.CCR-14-3370
A Phase I/II Trial of BNC105P with Everolimus in Metastatic Renal Cell Carcinoma
Abstract
Purpose: BNC105P inhibits tubulin polymerization, and preclinical studies suggest possible synergy with everolimus. In this phase I/II study, efficacy and safety of the combination were explored in patients with metastatic renal cell carcinoma (mRCC).
Experimental design: A phase I study in patients with clear cell mRCC and any prior number of therapies was conducted using a classical 3 + 3 design to evaluate standard doses of everolimus with increasing doses of BNC105P. At the recommended phase II dose (RP2D), patients with clear cell mRCC and one to two prior therapies (including ≥ 1 VEGF-TKI) were randomized to BNC105P with everolimus (arm A) or everolimus alone (arm B). The primary endpoint of the study was 6-month progression-free survival (6MPFS). Secondary endpoints included response rate, PFS, overall survival, and exploratory biomarker analyses.
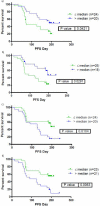
Results: In the phase I study (N = 15), a dose of BNC105P at 16 mg/m(2) with everolimus at 10 mg daily was identified as the RP2D. In the phase II study, 139 patients were randomized, with 69 and 67 evaluable patients in arms A and B, respectively. 6MPFS was similar in the treatment arms (arm A: 33.82% vs. arm B: 30.30%, P = 0.66) and no difference in median PFS was observed (arm A: 4.7 mos vs. arm B: 4.1 mos; P = 0.49). Changes in matrix metalloproteinase-9, stem cell factor, sex hormone-binding globulin, and serum amyloid A protein were associated with clinical outcome with BNC105P.
Conclusions: Although the primary endpoint was not met in an unselected population, correlative studies suggest several biomarkers that warrant further prospective evaluation.
©2015 American Association for Cancer Research.
Figures


References
-
- [March 21, 2014];National Comprehensive Cancer Network Clinical Practice Guidelines: Renal Cell Carcinoma. (Available at http://www.nccn.org.)
-
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. - PubMed
-
- Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. - PubMed
-
- Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, et al. Randomized Phase III Trial of Temsirolimus and Bevacizumab Versus Interferon Alfa and Bevacizumab in Metastatic Renal Cell Carcinoma: INTORACT Trial. J Clin Oncol. 2014;32:752–9. - PubMed
-
- Ravaud A, Barrios C, Anak Ö , Pelov D, Louveau A, Alekseev B, et al. Randomized phase II study of first-line everolimus (EVE) + bevacizumab (BEV) versus interferon alfa-2a (IFN) + BEV in patients (pts) with metastatic renal cell carcinoma (mRCC): RECORD-2. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23 [Abstr 7830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

