The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria
- PMID: 25788514
- PMCID: PMC4402952
- DOI: 10.1128/CMR.00117-14
The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria
Abstract
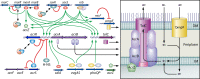
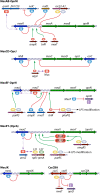
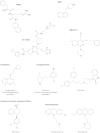
The global emergence of multidrug-resistant Gram-negative bacteria is a growing threat to antibiotic therapy. The chromosomally encoded drug efflux mechanisms that are ubiquitous in these bacteria greatly contribute to antibiotic resistance and present a major challenge for antibiotic development. Multidrug pumps, particularly those represented by the clinically relevant AcrAB-TolC and Mex pumps of the resistance-nodulation-division (RND) superfamily, not only mediate intrinsic and acquired multidrug resistance (MDR) but also are involved in other functions, including the bacterial stress response and pathogenicity. Additionally, efflux pumps interact synergistically with other resistance mechanisms (e.g., with the outer membrane permeability barrier) to increase resistance levels. Since the discovery of RND pumps in the early 1990s, remarkable scientific and technological advances have allowed for an in-depth understanding of the structural and biochemical basis, substrate profiles, molecular regulation, and inhibition of MDR pumps. However, the development of clinically useful efflux pump inhibitors and/or new antibiotics that can bypass pump effects continues to be a challenge. Plasmid-borne efflux pump genes (including those for RND pumps) have increasingly been identified. This article highlights the recent progress obtained for organisms of clinical significance, together with methodological considerations for the characterization of MDR pumps.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures








References
-
- World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

