Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning
- PMID: 25788518
- PMCID: PMC4553714
- DOI: 10.1093/hmg/ddv103
Elucidating the impact of neurofibromatosis-1 germline mutations on neurofibromin function and dopamine-based learning
Abstract
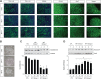
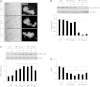
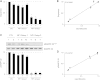
Neurofibromatosis type 1 (NF1) is a common autosomal dominant neurologic condition characterized by significant clinical heterogeneity, ranging from malignant cancers to cognitive deficits. Recent studies have begun to reveal rare genotype-phenotype correlations, suggesting that the specific germline NF1 gene mutation may be one factor underlying disease heterogeneity. The purpose of this study was to define the impact of the germline NF1 gene mutation on brain neurofibromin function relevant to learning. Herein, we employ human NF1-patient primary skin fibroblasts, induced pluripotent stem cells and derivative neural progenitor cells (NPCs) to demonstrate that NF1 germline mutations have dramatic effects on neurofibromin expression. Moreover, while all NF1-patient NPCs exhibit increased RAS activation and reduced cyclic AMP generation, there was a neurofibromin dose-dependent reduction in dopamine (DA) levels. Additionally, we leveraged two complementary Nf1 genetically-engineered mouse strains in which hippocampal-based learning and memory is DA-dependent to establish that neuronal DA levels and signaling as well as mouse spatial learning are controlled in an Nf1 gene dose-dependent manner. Collectively, this is the first demonstration that different germline NF1 gene mutations differentially dictate neurofibromin function in the brain.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Jett K., Friedman J.M. (2010) Clinical and genetic aspects of neurofibromatosis 1. Genet. Med., 12, 1–11. - PubMed
-
- Hyman S.L., Shores A., North K.N. (2005) The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology, 65, 1037–1044. - PubMed
-
- Isenberg J.C., Templer A., Gao F., Titus J.B., Gutmann D.H. (2013) Attention skills in children with neurofibromatosis type 1. J. Child Neurol., 28, 45–49. - PubMed
-
- Lorenzo J., Barton B., Arnold S.S., North K.N. (2013) Cognitive features that distinguish preschool-age children with neurofibromatosis type 1 from their peers: a matched case-control study. J. Pediatr., 163, 1479–1483 e1471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

