Akt Substrate of 160 kD Regulates Na+,K+-ATPase Trafficking in Response to Energy Depletion and Renal Ischemia
- PMID: 25788531
- PMCID: PMC4625659
- DOI: 10.1681/ASN.2013101040
Akt Substrate of 160 kD Regulates Na+,K+-ATPase Trafficking in Response to Energy Depletion and Renal Ischemia
Abstract
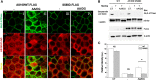
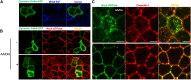
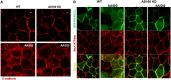
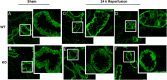
Renal ischemia and reperfusion injury causes loss of renal epithelial cell polarity and perturbations in tubular solute and fluid transport. Na(+),K(+)-ATPase, which is normally found at the basolateral plasma membrane of renal epithelial cells, is internalized and accumulates in intracellular compartments after renal ischemic injury. We previously reported that the subcellular distribution of Na(+),K(+)-ATPase is modulated by direct binding to Akt substrate of 160 kD (AS160), a Rab GTPase-activating protein that regulates the trafficking of glucose transporter 4 in response to insulin and muscle contraction. Here, we investigated the effect of AS160 on Na(+),K(+)-ATPase trafficking in response to energy depletion. We found that AS160 is required for the intracellular accumulation of Na(+),K(+)-ATPase that occurs in response to energy depletion in cultured epithelial cells. Energy depletion led to dephosphorylation of AS160 at S588, which was required for the energy depletion-induced accumulation of Na,K-ATPase in intracellular compartments. In AS160-knockout mice, the effects of renal ischemia on the distribution of Na(+),K(+)-ATPase were substantially reduced in the epithelial cells of distal segments of the renal tubules. These data demonstrate that AS160 has a direct role in linking the trafficking of Na(+),K(+)-ATPase to the energy state of renal epithelial cells.
Keywords: MDCK; cell biology; cell physiology; cell signaling; cell structure; epithelial; ischemia-reperfusion; transport physiology.
Copyright © 2015 by the American Society of Nephrology.
Figures



Similar articles
-
AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression.Mol Biol Cell. 2010 Dec;21(24):4400-8. doi: 10.1091/mbc.E10-06-0507. Epub 2010 Oct 13. Mol Biol Cell. 2010. PMID: 20943949 Free PMC article.
-
Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia.Am J Physiol Renal Physiol. 2011 Dec;301(6):F1346-57. doi: 10.1152/ajprenal.00420.2010. Epub 2011 Aug 17. Am J Physiol Renal Physiol. 2011. PMID: 21849490 Free PMC article.
-
Dissociation of spectrin-ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia.Am J Physiol Renal Physiol. 2003 Feb;284(2):F358-64. doi: 10.1152/ajprenal.00100.2002. Epub 2002 Oct 29. Am J Physiol Renal Physiol. 2003. PMID: 12409278
-
Role of the membrane-cytoskeleton in the spatial organization of the Na,K-ATPase in polarized epithelial cells.Soc Gen Physiol Ser. 1991;46:77-87. Soc Gen Physiol Ser. 1991. PMID: 1653995 Review.
-
Na+,K+ pump and Na+-coupled ion carriers in isolated mammalian kidney epithelial cells: regulation by protein kinase C.Can J Physiol Pharmacol. 1999 May;77(5):305-19. Can J Physiol Pharmacol. 1999. PMID: 10535680 Review.
Cited by
-
Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease.Int J Mol Sci. 2023 Apr 26;24(9):7855. doi: 10.3390/ijms24097855. Int J Mol Sci. 2023. PMID: 37175562 Free PMC article. Review.
-
Role of Na/K-ATPase α1 caveolin-binding motif in adipogenesis.Am J Physiol Cell Physiol. 2024 Jul 1;327(1):C48-C64. doi: 10.1152/ajpcell.00168.2024. Epub 2024 May 6. Am J Physiol Cell Physiol. 2024. PMID: 38708522 Free PMC article.
-
Ang II acutely stimulates Na,K-pump in cells from proximal tubules by increasing its phosphorylation at S938 via a PI3K/AKT pathway.Physiol Rep. 2022 Nov;10(21):e15508. doi: 10.14814/phy2.15508. Physiol Rep. 2022. PMID: 36377055 Free PMC article.
-
DR region specific antibody ameliorated but ouabain worsened renal injury in nephrectomized rats through regulating Na,K-ATPase mediated signaling pathways.Aging (Albany NY). 2019 Feb 26;11(4):1151-1162. doi: 10.18632/aging.101815. Aging (Albany NY). 2019. PMID: 30807290 Free PMC article.
-
Cardioprotective Effect of Cilostazol on Ischemia-Reperfusion Injury Model.Braz J Cardiovasc Surg. 2022 Dec 1;37(6):843-847. doi: 10.21470/1678-9741-2020-0651. Braz J Cardiovasc Surg. 2022. PMID: 34673517 Free PMC article.
References
-
- Jørgensen PL: Sodium and potassium ion pump in kidney tubules. Physiol Rev 60: 864–917, 1980 - PubMed
-
- Barlet-Bas C, Khadouri C, Marsy S, Doucet A: Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J Biol Chem 265: 7799–7803, 1990 - PubMed
-
- Horisberger JD, Rossier BC: Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension 19: 221–227, 1992 - PubMed
-
- Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ: Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282: 10585–10593, 2007 - PubMed
-
- Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM: Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and Is responsible for the decreased activity in epithelial cells. J Biol Chem 274: 1920–1927, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

