A Role for Tubular Necroptosis in Cisplatin-Induced AKI
- PMID: 25788533
- PMCID: PMC4625668
- DOI: 10.1681/ASN.2014080741
A Role for Tubular Necroptosis in Cisplatin-Induced AKI
Abstract
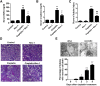
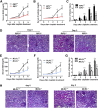
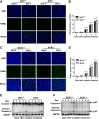
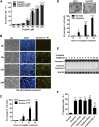
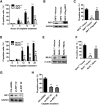
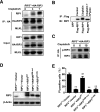
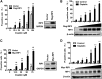
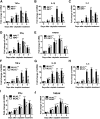
Cell death and inflammation in the proximal tubules are the hallmarks of cisplatin-induced AKI, but the mechanisms underlying these effects have not been fully elucidated. Here, we investigated whether necroptosis, a type of programmed necrosis, has a role in cisplatin-induced AKI. We found that inhibition of any of the core components of the necroptotic pathway-receptor-interacting protein 1 (RIP1), RIP3, or mixed lineage kinase domain-like protein (MLKL)-by gene knockout or a chemical inhibitor diminished cisplatin-induced proximal tubule damage in mice. Similar results were obtained in cultured proximal tubular cells. Furthermore, necroptosis of cultured cells could be induced by cisplatin or by a combination of cytokines (TNF-α, TNF-related weak inducer of apoptosis, and IFN-γ) that were upregulated in proximal tubules of cisplatin-treated mice. However, cisplatin induced an increase in RIP1 and RIP3 expression in cultured tubular cells in the absence of cytokine release. Correspondingly, overexpression of RIP1 or RIP3 enhanced cisplatin-induced necroptosis in vitro. Notably, inflammatory cytokine upregulation in cisplatin-treated mice was partially diminished in RIP3- or MLKL-deficient mice, suggesting a positive feedback loop involving these genes and inflammatory cytokines that promotes necroptosis progression. Thus, our data demonstrate that necroptosis is a major mechanism of proximal tubular cell death in cisplatin-induced nephrotoxic AKI.
Keywords: cisplatin nephrotoxicity; necroptosis; renal proximal tubule cell.
Copyright © 2015 by the American Society of Nephrology.
Figures

References
-
- Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 - PubMed
-
- Okada K, Ma D, Warabi E, Morito N, Akiyama K, Murata Y, Yamagata K, Bukawa H, Shoda J, Ishii T, Yanagawa T: Amelioration of cisplatin-induced nephrotoxicity in peroxiredoxin I-deficient mice. Cancer Chemother Pharmacol 71: 503–509, 2013 - PubMed
-
- Faubel S, Ljubanovic D, Reznikov L, Somerset H, Dinarello CA, Edelstein CL: Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int 66: 2202–2213, 2004 - PubMed
-
- Kim J, Long KE, Tang K, Padanilam BJ: Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int 82: 193–203, 2012 - PubMed
-
- Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S: Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

