Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage
- PMID: 25788671
- PMCID: PMC4363382
- DOI: 10.1523/JNEUROSCI.1188-14.2015
Regional and stage-specific effects of prospectively purified vascular cells on the adult V-SVZ neural stem cell lineage
Abstract
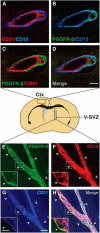
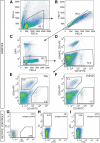
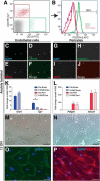
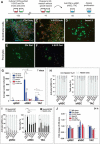
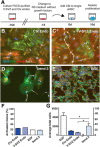
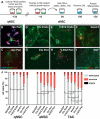
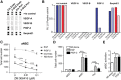
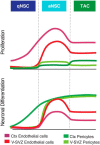
Adult neural stem cells reside in specialized niches. In the ventricular-subventricular zone (V-SVZ), quiescent neural stem cells (qNSCs) become activated (aNSCs), and generate transit amplifying cells (TACs), which give rise to neuroblasts that migrate to the olfactory bulb. The vasculature is an important component of the adult neural stem cell niche, but whether vascular cells in neurogenic areas are intrinsically different from those elsewhere in the brain is unknown. Moreover, the contribution of pericytes to the neural stem cell niche has not been defined. Here, we describe a rapid FACS purification strategy to simultaneously isolate primary endothelial cells and pericytes from brain microregions of nontransgenic mice using CD31 and CD13 as surface markers. We compared the effect of purified vascular cells from a neurogenic (V-SVZ) and non-neurogenic brain region (cortex) on the V-SVZ stem cell lineage in vitro. Endothelial and pericyte diffusible signals from both regions differentially promote the proliferation and neuronal differentiation of qNSCs, aNSCs, and TACs. Unexpectedly, diffusible cortical signals had the most potent effects on V-SVZ proliferation and neurogenesis, highlighting the intrinsic capacity of non-neurogenic vasculature to support stem cell behavior. Finally, we identify PlGF-2 as an endothelial-derived mitogen that promotes V-SVZ cell proliferation. This purification strategy provides a platform to define the functional and molecular contribution of vascular cells to stem cell niches and other brain regions under different physiological and pathological states.
Keywords: adult neurogenesis; endothelial cells; neural stem cell; niche; pericytes; vascular.
Copyright © 2015 the authors 0270-6474/15/354528-12$15.00/0.
Figures
References
-
- Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
