Selective activation of microglia facilitates synaptic strength
- PMID: 25788673
- PMCID: PMC4363384
- DOI: 10.1523/JNEUROSCI.2061-14.2015
Selective activation of microglia facilitates synaptic strength
Abstract
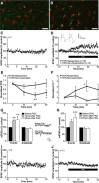
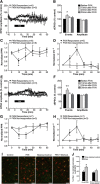
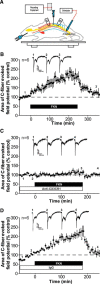
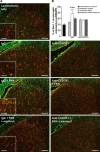
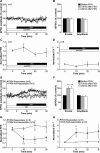
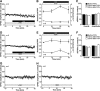
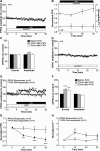
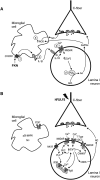
Synaptic plasticity is thought to be initiated by neurons only, with the prevailing view assigning glial cells mere specify supportive functions for synaptic transmission and plasticity. We now demonstrate that glial cells can control synaptic strength independent of neuronal activity. Here we show that selective activation of microglia in the rat is sufficient to rapidly facilitate synaptic strength between primary afferent C-fibers and lamina I neurons, the first synaptic relay in the nociceptive pathway. Specifically, the activation of the CX3CR1 receptor by fractalkine induces the release of interleukin-1β from microglia, which modulates NMDA signaling in postsynaptic neurons, leading to the release of an eicosanoid messenger, which ultimately enhances presynaptic neurotransmitter release. In contrast to the conventional view, this form of plasticity does not require enhanced neuronal activity to trigger the events leading to synaptic facilitation. Augmentation of synaptic strength in nociceptive pathways represents a cellular model of pain amplification. The present data thus suggest that, under chronic pain states, CX3CR1-mediated activation of microglia drives the facilitation of excitatory synaptic transmission in the dorsal horn, which contributes to pain hypersensitivity in chronic pain states.
Keywords: chemokine; chronic pain; fractalkine; microglia; spinal cord; synaptic plasticity.
Copyright © 2015 the authors 0270-6474/15/354552-19$15.00/0.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
