Nociceptor beta II, delta, and epsilon isoforms of PKC differentially mediate paclitaxel-induced spontaneous and evoked pain
- PMID: 25788678
- PMCID: PMC4363388
- DOI: 10.1523/JNEUROSCI.1580-14.2015
Nociceptor beta II, delta, and epsilon isoforms of PKC differentially mediate paclitaxel-induced spontaneous and evoked pain
Abstract
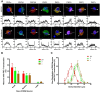
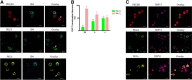
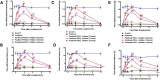
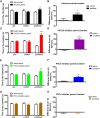
As one of the most effective and frequently used chemotherapeutic agents, paclitaxel produces peripheral neuropathy (paclitaxel-induced peripheral neuropathy or PIPN) that negatively affects chemotherapy and persists after cancer therapy. The mechanisms underlying this dose-limiting side effect remain to be fully elucidated. This study aimed to investigate the role of nociceptor protein kinase C (PKC) isoforms in PIPN. Employing multiple complementary approaches, we have identified a subset of PKC isoforms, namely βII, δ, and ϵ, were activated by paclitaxel in the isolated primary afferent sensory neurons. Persistent activation of PKCβII, PKCδ, and PKCϵ was also observed in the dorsal root ganglion neurons after chronic treatment with paclitaxel in a mouse model of PIPN. Isoform-selective inhibitors of PKCβII, PKCδ, and PKCϵ given intrathecally dose-dependently attenuated paclitaxel-induced mechanical allodynia and heat hyperalgesia. Surprisingly, spinal inhibition of PKCβII and PKCδ, but not PKCϵ, blocked the spontaneous pain induced by paclitaxel. These data suggest that a subset of nociceptor PKC isoforms differentially contribute to spontaneous and evoked pain in PIPN, although it is not clear whether PKCϵ in other regions regulates spontaneous pain in PIPN. The findings can potentially offer new selective targets for pharmacological intervention of PIPN.
Keywords: cancer; chemotherapy; pain; protein kinase C; taxane.
Copyright © 2015 the authors 0270-6474/15/354614-12$15.00/0.
Figures
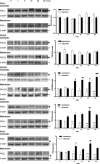
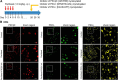
 (80 kDa) and
(80 kDa) and  (42 kDa).
(42 kDa).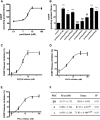
Comment in
-
Identifying the Role of Novel Protein Kinase C Isoforms in Mediating Paclitaxel-Induced Peripheral Neuropathy.J Neurosci. 2015 Jul 15;35(28):10101-2. doi: 10.1523/JNEUROSCI.1903-15.2015. J Neurosci. 2015. PMID: 26180187 Free PMC article. No abstract available.
References
-
- Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. - DOI - PMC - PubMed
-
- Barber LA, Vasko MR. Activation of protein kinase C augments peptide release from rat sensory neurons. J Neurochem. 1996;67:72–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
