Long-latency reductions in gamma power predict hemodynamic changes that underlie the negative BOLD signal
- PMID: 25788681
- PMCID: PMC4363390
- DOI: 10.1523/JNEUROSCI.2339-14.2015
Long-latency reductions in gamma power predict hemodynamic changes that underlie the negative BOLD signal
Abstract
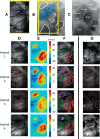
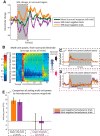
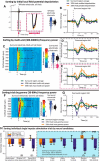
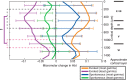
Studies that use prolonged periods of sensory stimulation report associations between regional reductions in neural activity and negative blood oxygenation level-dependent (BOLD) signaling. However, the neural generators of the negative BOLD response remain to be characterized. Here, we use single-impulse electrical stimulation of the whisker pad in the anesthetized rat to identify components of the neural response that are related to "negative" hemodynamic changes in the brain. Laminar multiunit activity and local field potential recordings of neural activity were performed concurrently with two-dimensional optical imaging spectroscopy measuring hemodynamic changes. Repeated measurements over multiple stimulation trials revealed significant variations in neural responses across session and animal datasets. Within this variation, we found robust long-latency decreases (300 and 2000 ms after stimulus presentation) in gamma-band power (30-80 Hz) in the middle-superficial cortical layers in regions surrounding the activated whisker barrel cortex. This reduction in gamma frequency activity was associated with corresponding decreases in the hemodynamic responses that drive the negative BOLD signal. These findings suggest a close relationship between BOLD responses and neural events that operate over time scales that outlast the initiating sensory stimulus, and provide important insights into the neurophysiological basis of negative neuroimaging signals.
Keywords: fMRI; gamma power; long latency; negative BOLD; neurovascular coupling; whisker barrel cortex.
Copyright © 2015 Boorman et al.
Figures



References
-
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. - PubMed
-
- Berwick J, Johnston D, Jones M, Martindale J, Martin C, Kennerley AJ, Redgrave P, Mayhew JE. Fine detail of neurovascular coupling revealed by spatiotemporal analysis of the hemodynamic response to single whisker stimulation in rat barrel cortex. J Neurophysiol. 2008;99:787–798. doi: 10.1152/jn.00658.2007. - DOI - PMC - PubMed
