Secreted frizzled related proteins modulate pathfinding and fasciculation of mouse retina ganglion cell axons by direct and indirect mechanisms
- PMID: 25788689
- PMCID: PMC6605131
- DOI: 10.1523/JNEUROSCI.3304-13.2015
Secreted frizzled related proteins modulate pathfinding and fasciculation of mouse retina ganglion cell axons by direct and indirect mechanisms
Abstract
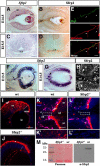
Retina ganglion cell (RGC) axons grow along a stereotyped pathway undergoing coordinated rounds of fasciculation and defasciculation, which are critical to establishing proper eye-brain connections. How this coordination is achieved is poorly understood, but shedding of guidance cues by metalloproteinases is emerging as a relevant mechanism. Secreted Frizzled Related Proteins (Sfrps) are multifunctional proteins, which, among others, reorient RGC growth cones by regulating intracellular second messengers, and interact with Tolloid and ADAM metalloproteinases, thereby repressing their activity. Here, we show that the combination of these two functions well explain the axon guidance phenotype observed in Sfrp1 and Sfrp2 single and compound mouse mutant embryos, in which RGC axons make subtle but significant mistakes during their intraretinal growth and inappropriately defasciculate along their pathway. The distribution of Sfrp1 and Sfrp2 in the eye is consistent with the idea that Sfrp1/2 normally constrain axon growth into the fiber layer and the optic disc. Disheveled axon growth instead seems linked to Sfrp-mediated modulation of metalloproteinase activity. Indeed, retinal explants from embryos with different Sfrp-null alleles or explants overexpressing ADAM10 extend axons with a disheveled appearance, which is reverted by the addition of Sfrp1 or an ADAM10-specific inhibitor. This mode of growth is associated with an abnormal proteolytic processing of L1 and N-cadherin, two ADAM10 substrates previously implicated in axon guidance. We thus propose that Sfrps contribute to coordinate visual axon growth with a dual mechanism: by directly signaling at the growth cone and by regulating the processing of other relevant cues.
Keywords: Sfrp; axon guidance; metalloproteinase; optic chiasm; optic disc; visual pathway.
Copyright © 2015 the authors 0270-6474/15/354729-12$15.00/0.
Figures






References
-
- Bastmeyer M, Ott H, Leppert CA, Stuermer CA. Fish E587 glycoprotein, a member of the L1 family of cell adhesion molecules, participates in axonal fasciculation and the age-related order of ganglion cell axons in the goldfish retina. J Cell Biol. 1995;130:969–976. doi: 10.1083/jcb.130.4.969. - DOI - PMC - PubMed
-
- Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–1241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
