Normal Growth of Healthy Infants Born from HIV+ Mothers Fed a Reduced Protein Infant Formula Containing the Prebiotics Galacto-Oligosaccharides and Fructo-Oligosaccharides: A Randomized Controlled Trial
- PMID: 25788839
- PMCID: PMC4357629
- DOI: 10.4137/CMPed.S17841
Normal Growth of Healthy Infants Born from HIV+ Mothers Fed a Reduced Protein Infant Formula Containing the Prebiotics Galacto-Oligosaccharides and Fructo-Oligosaccharides: A Randomized Controlled Trial
Abstract
Objective: The aim of the current study was to evaluate the safety of a new reduced protein (2.1 g/100 kcal) infant formula containing 4 g/L of 90% galacto-oligosaccharides (GOS) and 10% fructo-oligosaccharides (FOS).
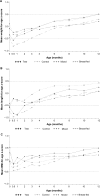
Methods: Healthy term infants from Brazil were enrolled. Those born to human immunodeficiency virus (HIV)-positive mothers were randomized to a test (n = 65) or control (n = 63) formula group. Infants born to HIV-negative mothers were either exclusively breast-fed (n = 79) or received a mixed diet (breast milk and test formula, n = 65). Between 2 weeks and 4 months of age, infants were exclusively fed according to their assigned group. Anthropometric measurements were taken at baseline, 1, 2, 3, 4, 6, 8, 10, and 12 months. Digestive tolerance was evaluated during the first 4 months. The primary outcome was mean daily weight gain between 2 weeks and 4 months in the test formula and breast-fed groups.
Results: Data from all infants (N = 272) were used in the intention-to-treat (ITT) analysis and data from 230 infants were used in the per-protocol (PP) analysis. The difference in mean daily weight gain between 2 weeks and 4 months in the test formula and breast-fed groups was 1.257 g/day (one-sided 95% confidence interval [CI]: -0.705 to inf, P < 0.001) in the PP analysis, showing that the lower bound of the 95% CI was above the -3.0 g/day non-inferiority margin. Results were similar in the ITT analysis. Symptoms of digestive tolerance and frequency of adverse events were similar in the two groups.
Conclusions: The formula containing 2.1 g/100 kcal protein and GOS and FOS was safe and tolerated well.
Keywords: fructo-oligosaccharides; galacto-oligosaccharides; infant formula; safety; weight gain.
Figures




References
-
- Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77(6):1537S–43S. - PubMed
-
- Mace K, Steenhout P, Klassen P, Donnet A. Protein quality and quantity in cow’s milk-based formula for healthy term infants: past, present and future. Nestle Nutr Workshop Ser Pediatr Program. 2006;58:189–203. discussion 203–185. - PubMed
-
- Räihä NC, Fazzolari-Nesci A, Cajozzo C, et al. Whey predominant, whey modified infant formula with protein/energy ratio of 1.8 g/100 kcal: adequate and safe for term infants from birth to four months. J Pediatr Gastroenterol Nutr. 2002;35(3):275–81. - PubMed
-
- Ziegler EE. Protein requirements in infancy. Infant formula: closer to the reference. In: Räihä N, Rubaltelli FF, editors. Nestlé Nutrition Workshop Series. Supplement Vol. 47. Philadelphia: Williams & Wilkins; 2002. pp. 97–110.
-
- Ziegler EE, Jeter JM, Drulis JM, Nelson SE, Haschke F, Steenhout P. Formula with reduced content of improved, partially hydrolyzed protein and probiotics: infant growth and health. Monatsschr Kinderheilkd. 2003;151:S65–71.
LinkOut - more resources
Full Text Sources
Miscellaneous

