Responsive organogels formed by supramolecular self assembly of PEG- block-allyl-functionalized racemic polypeptides into β-sheet-driven polymeric ribbons
- PMID: 25788968
- PMCID: PMC4361078
- DOI: 10.1039/C3SM50582K
Responsive organogels formed by supramolecular self assembly of PEG- block-allyl-functionalized racemic polypeptides into β-sheet-driven polymeric ribbons
Abstract
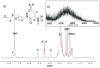
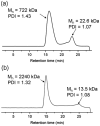
A chemically reactive hybrid diblock polypeptide gelator poly(ethylene glycol)-block-poly(dl-allylglycine) (PEG-b-PDLAG) is an exceptional material, due to the characteristics of thermo-reversible organogel formation driven by the combination of a hydrophilic polymer chain linked to a racemic oligomeric homopeptide segment in a range of organic solvents. One-dimensional stacking of the block copolymers is demonstrated by ATR-FTIR spectroscopy, wide-angle X-ray scattering to be driven by the supramolecular assembly of β-sheets in peptide blocks to afford well-defined fiber-like structures, resulting in gelation. These supramolecular interactions are sufficiently strong to achieve ultra low critical gelation concentrations (ca. 0.1 wt%) in N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and methanol. The critical gel transition temperature was directly proportional to the polymer concentration, so that at low concentrations, thermoreversibility of gelation was observed. Dynamic mechanical analysis studies were employed to determine the organogel mechanical properties, having storage moduli of ca. 15.1 kPa at room temperature.
Figures



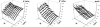
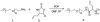

Similar articles
-
Tunable mechano-responsive organogels by ring-opening copolymerizations of N-carboxyanhydrides.Chem Sci. 2014 Jan;5(2):10.1039/C3SC52504J. doi: 10.1039/C3SC52504J. Chem Sci. 2014. PMID: 24363890 Free PMC article.
-
Erratum: Preparation of Poly(pentafluorophenyl acrylate) Functionalized SiO2 Beads for Protein Purification.J Vis Exp. 2019 Apr 30;(146). doi: 10.3791/6328. J Vis Exp. 2019. PMID: 31038480
-
Multi-responsive Hydrogels Derived from the Self-assembly of Tethered Allyl-functionalized Racemic Oligopeptides.J Mater Chem B. 2014 Dec 14;2(46):8123-8130. doi: 10.1039/C4TB00909F. J Mater Chem B. 2014. PMID: 25485113 Free PMC article.
-
Secondary Structure in Nonpeptidic Supramolecular Block Copolymers.Acc Chem Res. 2021 May 18;54(10):2397-2408. doi: 10.1021/acs.accounts.1c00028. Epub 2021 Apr 29. Acc Chem Res. 2021. PMID: 33914498 Review.
-
Thermo-induced physically crosslinked polypeptide-based block copolymer hydrogels for biomedical applications.Regen Biomater. 2023 Apr 20;10:rbad039. doi: 10.1093/rb/rbad039. eCollection 2023. Regen Biomater. 2023. PMID: 37265604 Free PMC article. Review.
Cited by
-
Tunable mechano-responsive organogels by ring-opening copolymerizations of N-carboxyanhydrides.Chem Sci. 2014 Jan;5(2):10.1039/C3SC52504J. doi: 10.1039/C3SC52504J. Chem Sci. 2014. PMID: 24363890 Free PMC article.
-
Design and Optimization of a Natural Medicine from Copaifera reticulata Ducke for Skin Wound Care.Polymers (Basel). 2022 Oct 23;14(21):4483. doi: 10.3390/polym14214483. Polymers (Basel). 2022. PMID: 36365477 Free PMC article.
-
Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose.Braz J Microbiol. 2017 Jan-Mar;48(1):180-185. doi: 10.1016/j.bjm.2016.11.001. Epub 2016 Nov 19. Braz J Microbiol. 2017. PMID: 27923548 Free PMC article.
-
Reversible photo-patterning of soft conductive materials via spatially-defined supramolecular assembly.Chem Commun (Camb). 2016 Jun 28;52(54):8455-8. doi: 10.1039/c6cc03579e. Chem Commun (Camb). 2016. PMID: 27305966 Free PMC article.
-
Transparent Organogels as a Medium for the Light-Induced Conversion from Spiropyran to Merocyanine.Gels. 2023 Nov 27;9(12):932. doi: 10.3390/gels9120932. Gels. 2023. PMID: 38131918 Free PMC article.
References
-
- Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Science. 2008;319:1812–1816. - PubMed
-
- Park MH, Joo MK, Choi BG, Jeong B. Acc Chem Res. 2012;45:424–433. - PubMed
-
- Kramer JR, Deming TJ. J Am Chem Soc. 2012;134:4112–4115. - PubMed
-
- Rodriguez-Hernandez J, Lecommandoux S. J Am Chem Soc. 2005;127:2026–2027. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

