Chronic Infection by Mucoid Pseudomonas aeruginosa Associated with Dysregulation in T-Cell Immunity to Outer Membrane Porin F
- PMID: 25789411
- PMCID: PMC4476516
- DOI: 10.1164/rccm.201411-1995OC
Chronic Infection by Mucoid Pseudomonas aeruginosa Associated with Dysregulation in T-Cell Immunity to Outer Membrane Porin F
Abstract
Rationale: Pseudomonas aeruginosa (PA) is an environmental pathogen that commonly infects individuals with cystic fibrosis (CF) and non-CF bronchiectasis, impacting morbidity and mortality. To understand the pathobiology of interactions between the bacterium and host adaptive immunity and to inform rational vaccine design, it is important to understand the adaptive immune correlates of disease.
Objectives: To characterize T-cell immunity to the PA antigen outer membrane porin F (OprF) by analyzing immunodominant epitopes in relation to infection status.
Methods: Patients with non-CF bronchiectasis were stratified by frequency of PA isolation. T-cell IFN-γ immunity to OprF and its immunodominant epitopes was characterized. Patterns of human leukocyte antigen (HLA) restriction of immunodominant epitopes were defined using HLA class II transgenic mice. Immunity was characterized with respect to cytokine and chemokine secretion, antibody response, and T-cell activation transcripts.
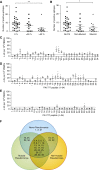
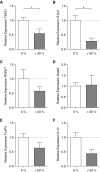
Measurements and main results: Patients were stratified according to whether PA was never, sometimes (<50%), or frequently (≥50%) isolated from sputum. Patients with frequent PA sputum-positive isolates were more likely to be infected by mucoid PA, and they showed a narrow T-cell epitope response and a relative reduction in Th1 polarizing transcription factors but enhanced immunity with respect to antibody production, innate cytokines, and chemokines.
Conclusions: We have defined the immunodominant, HLA-restricted T-cell epitopes of OprF. Our observation that chronic infection is associated with a response of narrowed specificity, despite strong innate and antibody immunity, may help to explain susceptibility in these individuals and pave the way for better vaccine design to achieve protective immunity.
Keywords: Pseudomonas; T lymphocyte; adaptive immunity; bronchiectasis; epitopes.
Figures



Similar articles
-
Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid.J Clin Invest. 2005 May;115(5):1281-9. doi: 10.1172/JCI23135. Epub 2005 Apr 1. J Clin Invest. 2005. PMID: 15841217 Free PMC article.
-
Protective anti-Pseudomonas aeruginosa humoral and cellular mucosal immunity by AdC7-mediated expression of the P. aeruginosa protein OprF.Vaccine. 2011 Mar 3;29(11):2131-9. doi: 10.1016/j.vaccine.2010.12.087. Epub 2011 Jan 6. Vaccine. 2011. PMID: 21215829 Free PMC article.
-
Protection against Pseudomonas aeruginosa lung infection in mice by recombinant OprF-pulsed dendritic cell immunization.BMC Microbiol. 2010 Jan 13;10:9. doi: 10.1186/1471-2180-10-9. BMC Microbiol. 2010. PMID: 20070893 Free PMC article.
-
Potential of protein OprF of Pseudomonas in bivalent vaccines.Behring Inst Mitt. 1997 Feb;(98):283-90. Behring Inst Mitt. 1997. PMID: 9382752 Review.
-
Rationale for development of immunotherapies that target mucoid Pseudomonas aeruginosa infection in cystic fibrosis patients.Behring Inst Mitt. 1997 Feb;(98):350-60. Behring Inst Mitt. 1997. PMID: 9382760 Review.
Cited by
-
Bioluminescent Reporting of In Vivo IFN-γ Immune Responses during Infection and Autoimmunity.J Immunol. 2019 Apr 15;202(8):2502-2510. doi: 10.4049/jimmunol.1801453. Epub 2019 Feb 27. J Immunol. 2019. PMID: 30814307 Free PMC article.
-
Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose.Science. 2021 Apr 30;372(6549):1418-23. doi: 10.1126/science.abh1282. Online ahead of print. Science. 2021. PMID: 33931567 Free PMC article.
-
Failed Eradication Therapy of New-Onset Pseudomonas aeruginosa Infections in Children With Cystic Fibrosis Is Associated With Bacterial Resistance to Neutrophil Functions.J Infect Dis. 2022 Jun 1;225(11):1886-1895. doi: 10.1093/infdis/jiab102. J Infect Dis. 2022. PMID: 33606875 Free PMC article.
-
Heterologous infection and vaccination shapes immunity against SARS-CoV-2 variants.Science. 2022 Jan 14;375(6577):183-192. doi: 10.1126/science.abm0811. Epub 2021 Dec 2. Science. 2022. PMID: 34855510 Free PMC article.
-
The Role of the Immune Response in the Pathogenesis of Bronchiectasis.Biomed Res Int. 2018 Mar 18;2018:6802637. doi: 10.1155/2018/6802637. eCollection 2018. Biomed Res Int. 2018. PMID: 29744361 Free PMC article. Review.
References
-
- Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology. 2010;15:1037–1056. - PubMed
-
- Govan JR, Brown AR, Jones AM. Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol. 2007;2:153–164. - PubMed
-
- Courtney JM, Bradley J, Mccaughan J, O’Connor TM, Shortt C, Bredin CP, Bradbury I, Elborn JS. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol. 2007;42:525–532. - PubMed
-
- Loebinger MR, Wells AU, Hansell DM, Chinyanganya N, Devaraj A, Meister M, Wilson R. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J. 2009;34:843–849. - PubMed
-
- May TB, Shinabarger D, Maharaj R, Kato J, Chu L, DeVault JD, Roychoudhury S, Zielinski NA, Berry A, Rothmel RK, et al. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991;4:191–206. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

