Sublingual immunization of trivalent human papillomavirus DNA vaccine in baculovirus nanovector for protection against vaginal challenge
- PMID: 25789464
- PMCID: PMC4366369
- DOI: 10.1371/journal.pone.0119408
Sublingual immunization of trivalent human papillomavirus DNA vaccine in baculovirus nanovector for protection against vaginal challenge
Abstract
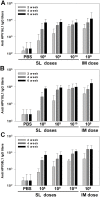
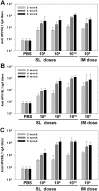
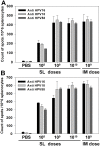
Here, we report the immunogenicity of a sublingually delivered, trivalent human papillomavirus (HPV) DNA vaccine encapsidated in a human endogenous retrovirus (HERV) envelope-coated, nonreplicable, baculovirus nanovector. The HERV envelope-coated, nonreplicable, baculovirus-based DNA vaccine, encoding HPV16L1, -18L1 and -58L1 (AcHERV-triHPV), was constructed and sublingually administered to mice without adjuvant. Following sublingual (SL) administration, AcHERV-triHPV was absorbed and distributed throughout the body. At 15 minutes and 1 day post-dose, the distribution of AcHERV-triHPV to the lung was higher than that to other tissues. At 30 days post-dose, the levels of AcHERV-triHPV had diminished throughout the body. Six weeks after the first of three doses, 1×10(8) copies of SL AcHERV-triHPV induced HPV type-specific serum IgG and neutralizing antibodies to a degree comparable to that of IM immunization with 1×10(9) copies. AcHERV-triHPV induced HPV type-specific vaginal IgA titers in a dose-dependent manner. SL immunization with 1×10(10) copies of AcHERV-triHPV induced Th1 and Th2 cellular responses comparable to IM immunization with 1×10(9) copies. Molecular imaging revealed that SL AcHERV-triHPV in mice provided complete protection against vaginal challenge with HPV16, HPV18, and HPV58 pseudoviruses. These results support the potential of SL immunization using multivalent DNA vaccine in baculovirus nanovector for induction of mucosal, systemic, and cellular immune responses.
Conflict of interest statement
Figures




Similar articles
-
Immunogenicity of a trivalent human papillomavirus L1 DNA-encapsidated, non-replicable baculovirus nanovaccine.PLoS One. 2014 Apr 23;9(4):e95961. doi: 10.1371/journal.pone.0095961. eCollection 2014. PLoS One. 2014. PMID: 24759938 Free PMC article.
-
Initial preclinical safety of non-replicating human endogenous retrovirus envelope protein-coated baculovirus vector-based vaccines against human papillomavirus.J Appl Toxicol. 2013 Dec;33(12):1474-83. doi: 10.1002/jat.2815. Epub 2012 Sep 14. J Appl Toxicol. 2013. PMID: 22987290
-
Immunogenicity of bivalent human papillomavirus DNA vaccine using human endogenous retrovirus envelope-coated baculoviral vectors in mice and pigs.PLoS One. 2012;7(11):e50296. doi: 10.1371/journal.pone.0050296. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209698 Free PMC article.
-
Development of a novel viral DNA vaccine against human papillomavirus: AcHERV-HP16L1.Vaccine. 2010 Feb 10;28(6):1613-9. doi: 10.1016/j.vaccine.2009.11.044. Epub 2009 Dec 2. Vaccine. 2010. PMID: 19961961
-
Baculovirus as vaccine vectors.Curr Gene Ther. 2010 Jun;10(3):201-13. doi: 10.2174/156652310791321233. Curr Gene Ther. 2010. PMID: 20394572 Review.
Cited by
-
Combined Subcutaneous-Intranasal Immunization With Epitope-Based Antigens Elicits Binding and Neutralizing Antibody Responses in Serum and Mucosae Against PRRSV-2 and SARS-CoV-2.Front Immunol. 2022 Mar 31;13:848054. doi: 10.3389/fimmu.2022.848054. eCollection 2022. Front Immunol. 2022. PMID: 35432364 Free PMC article.
-
Advances Towards Painless Vaccination and Newer Modes of Vaccine Delivery.Indian J Pediatr. 2018 Feb;85(2):132-138. doi: 10.1007/s12098-017-2377-2. Epub 2017 Jun 16. Indian J Pediatr. 2018. PMID: 28620730 Free PMC article. Review.
-
Microneedle-Mediated Vaccine Delivery to the Oral Mucosa.Adv Healthc Mater. 2019 Feb;8(4):e1801180. doi: 10.1002/adhm.201801180. Epub 2018 Dec 10. Adv Healthc Mater. 2019. PMID: 30537400 Free PMC article. Review.
-
Oral Coadministration of an Intramuscular DNA/Modified Vaccinia Ankara Vaccine for Simian Immunodeficiency Virus Is Associated with Better Control of Infection in Orally Exposed Infant Macaques.AIDS Res Hum Retroviruses. 2019 Mar;35(3):310-325. doi: 10.1089/AID.2018.0180. Epub 2018 Nov 27. AIDS Res Hum Retroviruses. 2019. PMID: 30303405 Free PMC article.
-
A simultaneous oral and intramuscular prime/sublingual boost with a DNA/Modified Vaccinia Ankara viral vector-based vaccine induces simian immunodeficiency virus-specific systemic and mucosal immune responses in juvenile rhesus macaques.J Med Primatol. 2018 Oct;47(5):288-297. doi: 10.1111/jmp.12372. Epub 2018 Sep 11. J Med Primatol. 2018. PMID: 30204253 Free PMC article.
References
-
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006; 6:148–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

