First administration of the Fc-attenuated anti-β amyloid antibody GSK933776 to patients with mild Alzheimer's disease: a randomized, placebo-controlled study
- PMID: 25789616
- PMCID: PMC4366075
- DOI: 10.1371/journal.pone.0098153
First administration of the Fc-attenuated anti-β amyloid antibody GSK933776 to patients with mild Alzheimer's disease: a randomized, placebo-controlled study
Abstract
Objective: To assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of the Fc-inactivated anti-β amyloid (Aβ) monoclonal antibody (mAb) GSK933776 in patients with mild Alzheimer's disease (AD) or mild cognitive impairment (MCI).
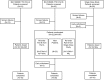
Methods: This was a two-part, single blind, placebo-controlled, first-time-in-human (FTIH) study of single (n = 18) and repeat dose (n = 32) intravenous GSK933776 0.001-6 mg/kg (ClinicalTrials.gov: NCT00459550). Additional safety data from an open-label, uncontrolled, single dose study of intravenous GSK933776 1-6 mg/kg (n = 18) are included (ClinicalTrials.gov: NCT01424436).
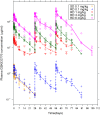
Results: There were no cases of amyloid-related imaging abnormalities-edema (ARIA-E) or -hemorrhage (ARIA-H) after GSK933776 administration in both studies. Three patients across the two studies developed anti-GSK933776 antibodies. Plasma GSK933776 half-life (t1/2) was 10-15 days after repeat dosing. After each of three administrations of GSK933776, plasma levels of total Aβ42 and Aβ increased whereas plasma levels of free Aβ decreased dose dependently; no changes were observed for placebo. For total Aβ42 the peak:trough ratio was ≤2 at doses ≥3 mg/kg; for total Aβ the ratio was ≤2 at 6 mg/kg. CSF concentrations of Aβ showed increases from baseline to week 12 for Aβ X-38 (week 12:baseline ratio: 1.65; 95%CI: 1.38, 1.93) and Aβ X-42 (week 12:baseline ratio: 1.18; 95%CI: 1.06, 1.30) for values pooled across doses.
Conclusion: In this FTIH study the Fc-inactivated anti-Aβ mAb GSK933776 engaged its target in plasma and CSF without causing brain ARIA-E/H in patients with mild AD or MCI.
Trial registration: ClinicalTrials.gov NCT00459550.
Conflict of interest statement
Figures

References
-
- Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, et al. (2010) 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 9: 363–372. 10.1016/S1474-4422(10)70043-0 - DOI - PubMed
-
- Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, et al. (2012) Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 69: 1002–1010. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

