Immunotherapeutic approaches for Alzheimer's disease
- PMID: 25789753
- PMCID: PMC4366618
- DOI: 10.1016/j.neuron.2014.12.064
Immunotherapeutic approaches for Alzheimer's disease
Abstract
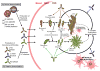
Alzheimer's disease (AD) is the most prevalent form of dementia worldwide and is an emerging global epidemic. It is characterized by an imbalance between production and clearance of amyloid β (Aβ) and tau proteins. Oligomeric forms of Aβ and tau are believed to be the most toxic. Dramatic results from AD animal models showed great promise for active and passive immune therapies targeting Aβ. However, there is very limited evidence in human studies of the clinical benefits from these approaches. Immunotherapies targeting only tau pathology have had some success but are limited so far to mouse models. The majority of current methods is based on immunological targeting of a self-protein; hence, benefits need to be balanced against risks of stimulating excessive autoimmune toxic inflammation. For greater efficacy the next generation of vaccines needs to focus more on concurrently targeting all the intermediate toxic conformers of oligomeric Aβ and tau species.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
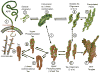
References
-
- Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J Neurosci. 2012;32:9677–9689. - PMC - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurol. 1992;42:631–639. - PubMed
-
- Asuni A, Boutajangout A, Scholtzova H, Knudsen E, Li Y, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Aβ derivative vaccination in alum adjuvant prevents amyloid deposition and does not cause brain microhemorrhages in Alzheimer’s model mice. Eur J Neurosci. 2006;24:2530–2542. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

