Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes
- PMID: 25789755
- PMCID: PMC4366619
- DOI: 10.1016/j.neuron.2015.02.026
Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes
Abstract
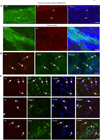
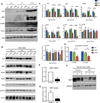
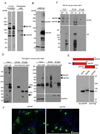
Growing evidence indicates that non-neuronal mutant huntingtin toxicity plays an important role in Huntington's disease (HD); however, whether and how mutant huntingtin affects oligodendrocytes, which are vitally important for neural function and axonal integrity, remains unclear. We first verified the presence of mutant huntingtin in oligodendrocytes in HD140Q knockin mice. We then established transgenic mice (PLP-150Q) that selectively express mutant huntingtin in oligodendrocytes. PLP-150Q mice show progressive neurological symptoms and early death, as well as age-dependent demyelination and reduced expression of myelin genes that are downstream of myelin regulatory factor (MYRF or MRF), a transcriptional regulator that specifically activates and maintains the expression of myelin genes in mature oligodendrocytes. Consistently, mutant huntingtin binds abnormally to MYRF and affects its transcription activity. Our findings suggest that dysfunction of mature oligodendrocytes is involved in HD pathogenesis and may also make a good therapeutic target.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Bartzokis G, Lu PH, Tishler TA, Fong SM, Oluwadara B, Finn JP, Huang D, Bordelon Y, Mintz J, Perlman S. Myelin breakdown and iron changes in Huntington's disease: pathogenesis and treatment implications. Neurochem Res. 2007;32:1655–1664. - PubMed
-
- Bohanna I, Georgiou-Karistianis N, Sritharan A, Asadi H, Johnston L, Churchyard A, Egan G. Diffusion tensor imaging in Huntington's disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav. 2011;5:171–180. - PubMed
-
- Boulanger JJ, Messier C. From precursors to myelinating oligodendrocytes: Contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain. Neuroscience. 2014;269C:343–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AG019206/AG/NIA NIH HHS/United States
- R01 NS041669/NS/NINDS NIH HHS/United States
- NS045016/NS/NINDS NIH HHS/United States
- MH/NS 31862/MH/NIMH NIH HHS/United States
- R01 NS095181/NS/NINDS NIH HHS/United States
- AG019206/AG/NIA NIH HHS/United States
- R01 NS045016/NS/NINDS NIH HHS/United States
- P50-AG025688/AG/NIA NIH HHS/United States
- P30 CA138292/CA/NCI NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- R01 NS036232/NS/NINDS NIH HHS/United States
- NS036232/NS/NINDS NIH HHS/United States
- P30CA138292/CA/NCI NIH HHS/United States
- R01 NS070526/NS/NINDS NIH HHS/United States
- AG31153/AG/NIA NIH HHS/United States
- NS041669/NS/NINDS NIH HHS/United States
- R01 AG019206/AG/NIA NIH HHS/United States
- R01 AG031153/AG/NIA NIH HHS/United States
- R01 NS056097/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

