Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch
- PMID: 25789756
- PMCID: PMC4372258
- DOI: 10.1016/j.neuron.2015.02.028
Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch
Abstract
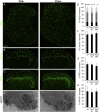
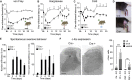
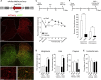
The gate control theory of pain proposes that inhibitory neurons of the spinal dorsal horn exert critical control over the relay of nociceptive signals to higher brain areas. Here we investigated how the glycinergic subpopulation of these neurons contributes to modality-specific pain and itch processing. We generated a GlyT2::Cre transgenic mouse line suitable for virus-mediated retrograde tracing studies and for spatially precise ablation, silencing, and activation of glycinergic neurons. We found that these neurons receive sensory input mainly from myelinated primary sensory neurons and that their local toxin-mediated ablation or silencing induces localized mechanical, heat, and cold hyperalgesia; spontaneous flinching behavior; and excessive licking and biting directed toward the corresponding skin territory. Conversely, local pharmacogenetic activation of the same neurons alleviated neuropathic hyperalgesia and chloroquine- and histamine-induced itch. These results establish glycinergic neurons of the spinal dorsal horn as key elements of an inhibitory pain and itch control circuit.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Glycine at the gate--from model to mechanism.Neuron. 2015 Mar 18;85(6):1152-4. doi: 10.1016/j.neuron.2015.03.012. Neuron. 2015. PMID: 25789751
References
-
- Ahmadi S., Lippross S., Neuhuber W.L., Zeilhofer H.U. PGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nat. Neurosci. 2002;5:34–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

