Membrane-active macromolecules resensitize NDM-1 gram-negative clinical isolates to tetracycline antibiotics
- PMID: 25789871
- PMCID: PMC4366164
- DOI: 10.1371/journal.pone.0119422
Membrane-active macromolecules resensitize NDM-1 gram-negative clinical isolates to tetracycline antibiotics
Erratum in
-
Correction: Membrane-active macromolecules resensitize NDM-1 gram-negative clinical isolates to tetracycline antibiotics.PLoS One. 2015 Apr 16;10(4):e0126757. doi: 10.1371/journal.pone.0126757. eCollection 2015. PLoS One. 2015. PMID: 25879927 Free PMC article.
Abstract
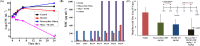
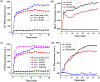
Gram-negative 'superbugs' such as New Delhi metallo-beta-lactamase-1 (blaNDM-1) producing pathogens have become world's major public health threats. Development of molecular strategies that can rehabilitate the 'old antibiotics' and halt the antibiotic resistance is a promising approach to target them. We report membrane-active macromolecules (MAMs) that restore the antibacterial efficacy (enhancement by >80-1250 fold) of tetracycline antibiotics towards blaNDM-1 Klebsiella pneumonia and blaNDM-1 Escherichia coli clinical isolates. Organismic studies showed that bacteria had an increased and faster uptake of tetracycline in the presence of MAMs which is attributed to the mechanism of re-sensitization. Moreover, bacteria did not develop resistance to MAMs and MAMs stalled the development of bacterial resistance to tetracycline. MAMs displayed membrane-active properties such as dissipation of membrane potential and membrane-permeabilization that enabled higher uptake of tetracycline in bacteria. In-vivo toxicity studies displayed good safety profiles and preliminary in-vivo antibacterial efficacy studies showed that mice treated with MAMs in combination with antibiotics had significantly decreased bacterial burden compared to the untreated mice. This report of re-instating the efficacy of the antibiotics towards blaNDM-1 pathogens using membrane-active molecules advocates their potential for synergistic co-delivery of antibiotics to combat Gram-negative superbugs.
Conflict of interest statement
Figures
Similar articles
-
Correction: Membrane-active macromolecules resensitize NDM-1 gram-negative clinical isolates to tetracycline antibiotics.PLoS One. 2015 Apr 16;10(4):e0126757. doi: 10.1371/journal.pone.0126757. eCollection 2015. PLoS One. 2015. PMID: 25879927 Free PMC article.
-
Vancomycin Analogue Restores Meropenem Activity against NDM-1 Gram-Negative Pathogens.ACS Infect Dis. 2018 Jul 13;4(7):1093-1101. doi: 10.1021/acsinfecdis.8b00011. Epub 2018 May 14. ACS Infect Dis. 2018. PMID: 29726673
-
A Common Flanking Region in Promiscuous Plasmids Encoding blaNDM-1 in Klebsiella pneumoniae Isolated in Singapore.Microb Drug Resist. 2016 Mar;22(2):109-14. doi: 10.1089/mdr.2015.0132. Epub 2015 Aug 26. Microb Drug Resist. 2016. PMID: 26308279
-
The present danger of New Delhi metallo-β-lactamase: a threat to public health.Future Microbiol. 2020 Dec;15(18):1759-1778. doi: 10.2217/fmb-2020-0069. Epub 2021 Jan 6. Future Microbiol. 2020. PMID: 33404261 Review.
-
[NDM-1: the superbug?].Infez Med. 2011 Dec;19(4):224-34. Infez Med. 2011. PMID: 22212161 Review. Italian.
Cited by
-
Facially Amphiphilic Bile Acid-Functionalized Antimicrobials: Combating Pathogenic Bacteria, Fungi, and Their Biofilms.ACS Infect Dis. 2023 Sep 8;9(9):1769-1782. doi: 10.1021/acsinfecdis.3c00266. Epub 2023 Aug 3. ACS Infect Dis. 2023. PMID: 37535907 Free PMC article.
-
Diversity of International High-Risk Clones of Acinetobacter baumannii Revealed in a Russian Multidisciplinary Medical Center during 2017-2019.Antibiotics (Basel). 2021 Aug 20;10(8):1009. doi: 10.3390/antibiotics10081009. Antibiotics (Basel). 2021. PMID: 34439060 Free PMC article.
-
Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance.ACS Omega. 2023 Mar 14;8(12):10757-10783. doi: 10.1021/acsomega.3c00312. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008128 Free PMC article. Review.
-
Correction: Membrane-active macromolecules resensitize NDM-1 gram-negative clinical isolates to tetracycline antibiotics.PLoS One. 2015 Apr 16;10(4):e0126757. doi: 10.1371/journal.pone.0126757. eCollection 2015. PLoS One. 2015. PMID: 25879927 Free PMC article.
-
NDM Metallo-β-Lactamases and Their Bacterial Producers in Health Care Settings.Clin Microbiol Rev. 2019 Jan 30;32(2):e00115-18. doi: 10.1128/CMR.00115-18. Print 2019 Mar 20. Clin Microbiol Rev. 2019. PMID: 30700432 Free PMC article. Review.
References
-
- Antimicrobial resistance: global report on surveillance. World health Organization; Geneva: 2014. Available: http://www.who.int/drugresistance/documents/surveillancereport/en.pdf. Accessed 2014 May 9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

