Palladium-catalyzed α-arylation of carbonyls in the de novo synthesis of aromatic heterocycles
- PMID: 25789887
- PMCID: PMC4424829
- DOI: 10.1039/c5ob00055f
Palladium-catalyzed α-arylation of carbonyls in the de novo synthesis of aromatic heterocycles
Abstract
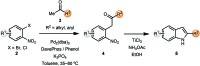
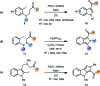
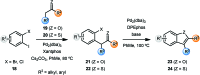
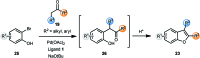
Aromatic heterocycles are a very well represented motif in natural products and have found various applications in chemistry and material science, as well as being commonly found in pharmaceutical agents. Thus, new and efficient routes towards this class of compound are always desirable, particularly if they expand the scope of chemical methodology or facilitate more effective pathways to complex substitution patterns. This perspective covers recent developments in the de novo synthesis of aromatic heterocycles via palladium-catalysed α-arylation reactions of carbonyls, which is itself a powerful transformation that has undergone significant development in recent years.
Figures



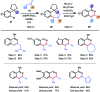
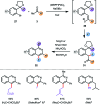
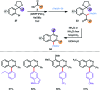
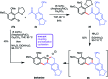
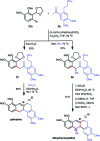



References
-
-
. For selected reviews, see:
- Palucki M., Buchwald S. L. J. Am. Chem. Soc. 1997;119:11108–11109.
- Hamann B. C., Hartwig J. F. J. Am. Chem. Soc. 1997;119:12382–12383.
- Satoh T., Kawamura Y., Miura M., Nomura M. Angew. Chem., Int. Ed. 1997;36:1740–1742.
- Åhman J., Wolfe J. P., Troutman M. V., Palucki M., Buchwald S. L. J. Am. Chem. Soc. 1998;120:1918–1919.
- Shaughnessy K. H., Hamann B. C., Hartwig J. F. J. Org. Chem. 1998;63:6546–6553.
- Kawatsura M., Hartwig J. F. J. Am. Chem. Soc. 1999;121:1473–1478.
- Fox J. M., Huang X., Chieffi A., Buchwald S. L. J. Am. Chem. Soc. 2000;122:1360–1370.
- Stauffer S. R., Beare N. A., Stambuli J. P., Hartwig J. F. J. Am. Chem. Soc. 2001;123:4641–4642. - PubMed
- Moradi W. A., Buchwald S. L. J. Am. Chem. Soc. 2001;123:7996–8002. - PubMed
- Lee S., Beare N. A., Hartwig J. F. J. Am. Chem. Soc. 2001;123:8410–8411. - PubMed
- Wolkowski J. P., Hartwig J. F. Angew. Chem., Int. Ed. 2002;41:4289–4291. - PubMed
- Beare N. A., Hartwig J. F. J. Org. Chem. 2002;67:541–555. - PubMed
- Bellina F., Rossi R. Chem. Rev. 2010;110:1082–1146. - PubMed
- Johansson C. C. C., Colacot T. J. Angew. Chem., Int. Ed. 2010;49:676–707. - PubMed
- Sivanandan S. T., Shaji A., Ibnusaud I., Seechurn C. C. C. J., Colacot T. J. Eur. J. Org. Chem. 2015;2015:38–49.
-
-
- Rutherford J. L., Rainka M. P., Buchwald S. L. J. Am. Chem. Soc. 2002;124:15168–15169. - PubMed
-
- Solé D., Vallverdú L., Peidró E., Bonjoch J. Chem. Commun. 2001:1888–1889. - PubMed
- Solé D., Vallverdú L., Solans X., Bardía M. F., Bonjoch J. J. Am. Chem. Soc. 2003;125:1587–1594. - PubMed
- Solé D., Serrano O. J. Org. Chem. 2008;73:2476–2479. - PubMed
- Solé D., Serrano O. J. Org. Chem. 2008;73:9372–9378. - PubMed
- Solé D., Serrano O. Org. Biomol. Chem. 2009;7:3382–3384. - PubMed
- Solé D., Fernández I., Sierra M. A. Chem. – Eur. J. 2012;18:6950–6958. - PubMed
- Solé D., Serrano O. J. Org. Chem. 2010;75:6267–6270. - PubMed
- Solé D., Bennasar M.-L., Jiménez I. Org. Biomol. Chem. 2011;9:4535–4544. - PubMed
- Fernández I., Solé D., Sierra M. A. J. Org. Chem. 2011;76:1592–1598. - PubMed
- Solé D., Fernández I. Acc. Chem. Res. 2014;47:168–179. - PubMed
-
- Terao Y., Satoh T., Miura M., Nomura M. Bull. Chem. Soc. Jpn. 1999;72:2345–2350.
LinkOut - more resources
Full Text Sources
Other Literature Sources

