Network meta-analysis of randomized controlled trials: efficacy and safety of UDCA-based therapies in primary biliary cirrhosis
- PMID: 25789951
- PMCID: PMC4602497
- DOI: 10.1097/MD.0000000000000609
Network meta-analysis of randomized controlled trials: efficacy and safety of UDCA-based therapies in primary biliary cirrhosis
Abstract
Major ursodeoxycholic acid (UDCA)-based therapies for primary biliary cirrhosis (PBC) include UDCA only, or combined with either methotrexate (MTX), corticosteroids (COT), colchicine (COC), or bezafibrate (BEF). As the optimum treatment regimen is unclear and warrants exploration, we aimed to compare these therapies in terms of patient mortality or liver transplantation (MOLT) and adverse events (AE).PubMed, the Cochrane Library, and Scopus were searched for randomized controlled trials up to August 31, 2014. We estimated the hazard ratios (HRs) for MOLT and odds ratios (ORs) for AE. A sensitivity analysis based on the dose of UDCA was also executed.Thirty-one eligible articles were included. Compared with COT plus UDCA, UDCA (HR 0.38, 95% confidence interval [CI] 0.09-1.39), BEF plus UDCA (HR 0.29, 95% CI 0.02-4.83), COC plus UDCA (HR 0.39, 95% CI 0.07-2.25), MTX plus UDCA (HR 0.28, 95% CI 0.05-1.63), or OBS (HR 0.49, 95% CI 0.11-2.01) all provided an increased risk of MOLT. With respect to drug AE profile, although not differing appreciably, BEF plus UDCA was associated with more AEs compared with UDCA (OR 3.16, 95% CI 0.59-20.67), COT plus UDCA (OR 2.27, 95% CI 0.15-33.36), COC plus UDCA (OR 1.00, 95% CI 0.09-12.16), MTX plus UDCA (OR 2.03, 95% CI 0.23-17.82), or OBS (OR 3.00, 95% CI 0.53-20.75). The results of sensitivity analyses were highly consistent with previous analyses.COT plus UDCA was the optimal UDCA-based regimen for both MOLT and AEs. BEF plus UDCA was most likely to cause AEs, whereas monotherapy with UDCA and coadministriation of COT plus UDCA appeared to be associated with the fewest AEs for PBC treatment.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
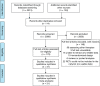




References
-
- Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet 2003; 362:53–61. - PubMed
-
- Kaplan MM, Cheng S, Price LL, et al. A randomized controlled trial of colchicine plus ursodiol versus methotrexate plus ursodiol in primary biliary cirrhosis: ten-year results. Hepatology (Baltimore, MD) 2004; 39:915–923. - PubMed
-
- Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology (Baltimore, MD) 2008; 48:871–877. - PubMed
-
- Heathcote EJ, Cauch-Dudek K, Walker V, et al. The Canadian Multicenter Double-blind Randomized Controlled Trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology (Baltimore, MD) 1994; 19:1149–1156. - PubMed
-
- Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology 1994; 106:1284–1290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

