A pilot study of omalizumab in eosinophilic esophagitis
- PMID: 25789989
- PMCID: PMC4366078
- DOI: 10.1371/journal.pone.0113483
A pilot study of omalizumab in eosinophilic esophagitis
Abstract
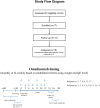
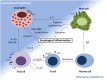
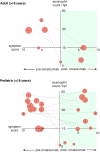
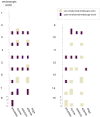
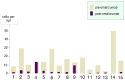
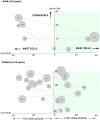
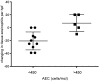
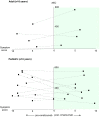
Eosinophilic disorders of the gastrointestinal tract are an emerging subset of immune pathologies within the spectrum of allergic inflammation. Eosinophilic Esophagitis (EoE), once considered a rare disease, is increasing in incidence, with a rate of over 1 in 10,000 in the US, for unknown reasons. The clinical management of EoE is challenging, thus there is an urgent need for understanding the etiology and pathophysiology of this eosinophilic disease to develop better therapeutic approaches. In this open label, single arm, unblinded study, we evaluated the effects of an anti-IgE treatment, omalizumab, on local inflammation in the esophagus and clinical correlates in patients with EoE. Omalizumab was administered for 12 weeks to 15 subjects with long standing EoE. There were no serious side effects from the treatment. Esophageal tissue inflammation was assessed both before and after therapy. After 3 months on omalizumab, although tissue Immunoglobulin E (IgE) levels were significantly reduced in all but two of the subjects, we found that full remission of EoE, which is defined as histologic and clinical improvement only in 33% of the patients. The decrease in tryptase-positive cells and eosinophils correlated significantly with the clinical outcome as measured by improvement in endoscopy and symptom scores, respectively. Omalizumab-induced remission of EoE was limited to subjects with low peripheral blood absolute eosinophil counts. These findings demonstrate that in a subset of EoE patients, IgE plays a role in the pathophysiology of the disease and that anti-IgE therapy with omalizumab may result in disease remission. Since this study is open label there is the potential for bias, hence the need for a larger double blind placebo controlled study. The data presented in this pilot study provides a foundation for proper patient selection to maximize clinical efficacy.
Trial registration: ClinicalTrials.gov NCT01040598.
Conflict of interest statement
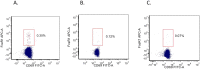
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

