IL-4Rα-dependent alternative activation of macrophages is not decisive for Mycobacterium tuberculosis pathology and bacterial burden in mice
- PMID: 25790379
- PMCID: PMC4366092
- DOI: 10.1371/journal.pone.0121070
IL-4Rα-dependent alternative activation of macrophages is not decisive for Mycobacterium tuberculosis pathology and bacterial burden in mice
Abstract
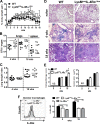
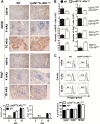
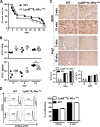
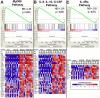
Classical activation of macrophages (caMph or M1) is crucial for host protection against Mycobacterium tuberculosis (Mtb) infection. Evidence suggests that IL-4/IL-13 alternatively activated macrophages (aaMph or M2) are exploited by Mtb to divert microbicidal functions of caMph. To define the functions of M2 macrophages during tuberculosis (TB), we infected mice deficient for IL-4 receptor α on macrophages (LysMcreIL-4Rα-/lox) with Mtb. We show that absence of IL-4Rα on macrophages does not play a major role during infection with Mtb H37Rv, or the clinical Beijing strain HN878. This was demonstrated by similar mortality, bacterial burden, histopathology and T cell proliferation between infected wild-type (WT) and LysMcreIL-4Rα-/lox mice. Interestingly, we observed no differences in the lung expression of inducible nitric oxide synthase (iNOS) and Arginase 1 (Arg1), well-established markers for M1/M2 macrophages among the Mtb-infected groups. Kinetic expression studies of IL-4/IL-13 activated bone marrow-derived macrophages (BMDM) infected with HN878, followed by gene set enrichment analysis, revealed that the MyD88 and IL-6, IL-10, G-CSF pathways are significantly enriched, but not the IL-4Rα driven pathway. Together, these results suggest that IL-4Rα-macrophages do not play a central role in TB disease progression.
Conflict of interest statement
Figures

References
-
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008. September 15;181(6):3733–9. - PubMed
-
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003. January;3(1):23–35. - PubMed
-
- Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol. 2007. June;8(6):610–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

