Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance
- PMID: 25791085
- PMCID: PMC4710357
- DOI: 10.1126/science.aaa7017
Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance
Abstract
Aire is an important regulator of immunological tolerance, operating in a minute subset of thymic stromal cells to induce transcripts encoding peptides that guide T cell selection. Expression of Aire during a perinatal age window is necessary and sufficient to prevent the multiorgan autoimmunity characteristic of Aire-deficient mice. We report that Aire promotes the perinatal generation of a distinct compartment of Foxp3(+)CD4(+) regulatory T (Treg) cells, which stably persists in adult mice. This population has a role in maintaining self-tolerance, a transcriptome and an activation profile distinguishable from those of Tregs produced in adults. Underlying the distinct Treg populations are age-dependent, Aire-independent differences in the processing and presentation of thymic stromal-cell peptides, resulting in different T cell receptor repertoires. Our findings expand the notion of a developmentally layered immune system.
Copyright © 2015, American Association for the Advancement of Science.
Figures
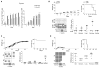

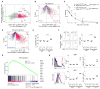
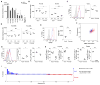
Comment in
-
Regulatory T cells: Young AIREs go on to rule.Nat Rev Immunol. 2015 May;15(5):269. doi: 10.1038/nri3849. Epub 2015 Apr 7. Nat Rev Immunol. 2015. PMID: 25848756 No abstract available.
-
Immunology. Early life Aire.Science. 2015 May 1;348(6234):506-7. doi: 10.1126/science.aab2998. Epub 2015 Apr 30. Science. 2015. PMID: 25931543 No abstract available.
References
-
- Aschenbrenner K, et al. Selection of Foxp3(+) regulatory T cells specific for self antigen expressed and presented by Aire(+) medullary thymic epithelial cells. Nat Immunol. 2007;8:351. - PubMed
-
- Hinterberger M, et al. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11:512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

