Development of animal models against emerging coronaviruses: From SARS to MERS coronavirus
- PMID: 25791336
- PMCID: PMC4793273
- DOI: 10.1016/j.virol.2015.02.030
Development of animal models against emerging coronaviruses: From SARS to MERS coronavirus
Abstract
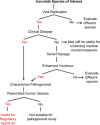
Two novel coronaviruses have emerged to cause severe disease in humans. While bats may be the primary reservoir for both viruses, SARS coronavirus (SARS-CoV) likely crossed into humans from civets in China, and MERS coronavirus (MERS-CoV) has been transmitted from camels in the Middle East. Unlike SARS-CoV that resolved within a year, continued introductions of MERS-CoV present an on-going public health threat. Animal models are needed to evaluate countermeasures against emerging viruses. With SARS-CoV, several animal species were permissive to infection. In contrast, most laboratory animals are refractory or only semi-permissive to infection with MERS-CoV. This host-range restriction is largely determined by sequence heterogeneity in the MERS-CoV receptor. We describe animal models developed to study coronaviruses, with a focus on host-range restriction at the level of the viral receptor and discuss approaches to consider in developing a model to evaluate countermeasures against MERS-CoV.
Keywords: Animal models; Coronaviruses; MERS-CoV; Receptor; SARS-CoV.
Copyright © 2015. Published by Elsevier Inc.
Figures
References
-
- Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370(26):2499–2505. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

