Oligomerized, filamentous surface presentation of RANTES/CCL5 on vascular endothelial cells
- PMID: 25791723
- PMCID: PMC4367157
- DOI: 10.1038/srep09261
Oligomerized, filamentous surface presentation of RANTES/CCL5 on vascular endothelial cells
Abstract
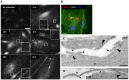
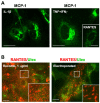
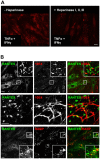
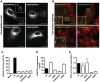
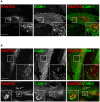
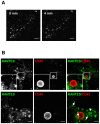
Vascular endothelial cells present luminal chemokines that arrest rolling leukocytes by activating integrins. It appears that several chemokines must form higher-order oligomers to elicit proper in vivo effects, as mutants restricted to forming dimers have lost the ability to recruit leukocytes to sites of inflammation. Here, we show for the first time that the chemokine RANTES/CCL5 binds to the surface of human endothelial cells in a regular filamentous pattern. Furthermore, the filaments bound to the surface in a heparan sulfate-dependent manner. By electron microscopy we observed labeling for RANTES on membrane projections as well as on the remaining plasma membrane. Mutant constructs of RANTES restricted either in binding to heparin, or in forming dimers or tetramers, appeared either in a granular, non-filamentous pattern or were not detectable on the cell surface. The RANTES filaments were also present after exposure to flow, suggesting that they can be present in vivo. Taken together with the lacking in vivo or in vitro effects of RANTES mutants, we suggest that the filamentous structures of RANTES may be of physiological importance in leukocyte recruitment.
Figures

Similar articles
-
Chondroitin sulfate A released from platelets blocks RANTES presentation on cell surfaces and RANTES-dependent firm adhesion of leukocytes.Eur J Immunol. 2002 Apr;32(4):1012-20. doi: 10.1002/1521-4141(200204)32:4<1012::AID-IMMU1012>3.0.CO;2-T. Eur J Immunol. 2002. PMID: 11920567
-
Oligomerization of RANTES is required for CCR1-mediated arrest but not CCR5-mediated transmigration of leukocytes on inflamed endothelium.Blood. 2003 Sep 15;102(6):1985-8. doi: 10.1182/blood-2003-04-1175. Epub 2003 May 22. Blood. 2003. PMID: 12763925
-
Endothelial inflammation: the role of differential expression of N-deacetylase/N-sulphotransferase enzymes in alteration of the immunological properties of heparan sulphate.J Cell Sci. 2003 Sep 1;116(Pt 17):3591-600. doi: 10.1242/jcs.00662. Epub 2003 Jul 22. J Cell Sci. 2003. PMID: 12876215
-
Angiogenic properties of the chemokine RANTES/CCL5.Biochem Soc Trans. 2011 Dec;39(6):1649-53. doi: 10.1042/BST20110651. Biochem Soc Trans. 2011. PMID: 22103502 Review.
-
Some aspects of IL-8 pathophysiology. III: Chemokine interaction with endothelial cells.J Leukoc Biol. 1996 Jan;59(1):39-44. doi: 10.1002/jlb.59.1.39. J Leukoc Biol. 1996. PMID: 8558065 Review.
Cited by
-
Potential Role of Antioxidant and Anti-Inflammatory Therapies to Prevent Severe SARS-Cov-2 Complications.Antioxidants (Basel). 2021 Feb 10;10(2):272. doi: 10.3390/antiox10020272. Antioxidants (Basel). 2021. PMID: 33578849 Free PMC article. Review.
-
Lactobacillus plantarum displaying CCL3 chemokine in fusion with HIV-1 Gag derived antigen causes increased recruitment of T cells.Microb Cell Fact. 2015 Oct 22;14:169. doi: 10.1186/s12934-015-0360-z. Microb Cell Fact. 2015. PMID: 26494531 Free PMC article.
-
Migration arrest and transendothelial trafficking of human pathogenic-like Th17 cells are mediated by differentially positioned chemokines.Nat Commun. 2025 Feb 26;16(1):1978. doi: 10.1038/s41467-025-57002-6. Nat Commun. 2025. PMID: 40000641 Free PMC article.
-
Encompassing view of spatial and single-cell RNA sequencing renews the role of the microvasculature in human atherosclerosis.Nat Cardiovasc Res. 2025 Jan;4(1):26-44. doi: 10.1038/s44161-024-00582-1. Epub 2024 Dec 23. Nat Cardiovasc Res. 2025. PMID: 39715784
-
Preventing the development of severe COVID-19 by modifying immunothrombosis.Life Sci. 2021 Jan 1;264:118617. doi: 10.1016/j.lfs.2020.118617. Epub 2020 Oct 20. Life Sci. 2021. PMID: 33096114 Free PMC article. Review.
References
-
- Shamri R. et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol 6, 497–506 (2005). - PubMed
-
- Bonecchi R. et al. Chemokines and chemokine receptors: an overview. Front Biosci (Landmark Ed) 14, 540–51 (2009). - PubMed
-
- Anders H. J., Romagnani P. & Mantovani A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol Med 20, 154–65 (2014). - PubMed
-
- Griffith J. W., Sokol C. L. & Luster A. D. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32, 659–702 (2014). - PubMed
-
- Hoogewerf A. J. et al. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry 36, 13570–8 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

