Melanoma brain metastasis is independent of lactate dehydrogenase A expression
- PMID: 25791837
- PMCID: PMC4578581
- DOI: 10.1093/neuonc/nov040
Melanoma brain metastasis is independent of lactate dehydrogenase A expression
Abstract
Background: The key metabolic enzyme lactate dehydrogenase A (LDHA) is overexpressed in many cancers, and several preclinical studies have shown encouraging results of targeted inhibition. However, the mechanistic importance of LDHA in melanoma is largely unknown and hitherto unexplored in brain metastasis.
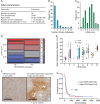
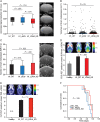
Methods: We investigated the spatial, temporal, and functional features of LDHA expression in melanoma brain metastasis across multiple in vitro assays, in a robust and predictive animal model employing MRI and PET imaging, and in a unique cohort of 80 operated patients. We further assessed the genomic and proteomic landscapes of LDHA in different cancers, particularly melanomas.
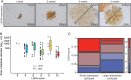
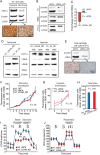
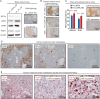
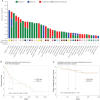
Results: LDHA expression was especially strong in early and small brain metastases in vivo and related to intratumoral hypoxia in late and large brain metastases in vivo and in patients. However, LDHA expression in human brain metastases was not associated with the number of tumors, BRAF(V600E) status, or survival. Moreover, LDHA depletion by small hairpin RNA interference did not affect cell proliferation or 3D tumorsphere growth in vitro or brain metastasis formation or survival in vivo. Integrated analyses of the genomic and proteomic landscapes of LDHA indicated that LDHA is present but not imperative for tumor progression within the CNS, or predictive of survival in melanoma patients.
Conclusions: In a large patient cohort and in a robust animal model, we show that although LDHA expression varies biphasically during melanoma brain metastasis formation, tumor progression and survival seem to be functionally independent of LDHA.
Keywords: LDHA; brain metastasis; lactate dehydrogenase A; melanoma; metabolism.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Eggermont AM, Spatz A, Robert C. Cutaneous melanoma. Lancet. 2014;383(9919):816–827. - PubMed
-
- Flanigan JC, Jilaveanu LB, Chiang VL, et al. Advances in therapy for melanoma brain metastases. Clin Dermatol. 2013;31(3):264–281. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

