Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment
- PMID: 25792100
- PMCID: PMC4520251
- DOI: 10.1016/S1474-4422(14)70201-7
Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment
Erratum in
-
Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment.Lancet Neurol. 2015 May;14(5):461. doi: 10.1016/S1474-4422(15)00010-1. Epub 2015 Mar 26. Lancet Neurol. 2015. PMID: 25895926 No abstract available.
Abstract
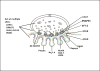
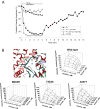
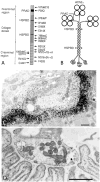
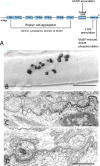
The congenital myasthenic syndromes (CMS) are a diverse group of genetic disorders caused by abnormal signal transmission at the motor endplate, a special synaptic contact between motor axons and each skeletal muscle fibre. Most CMS stem from molecular defects in the muscle nicotinic acetylcholine receptor, but they can also be caused by mutations in presynaptic proteins, mutations in proteins associated with the synaptic basal lamina, defects in endplate development and maintenance, or defects in protein glycosylation. The specific diagnosis of some CMS can sometimes be reached by phenotypic clues pointing to the mutated gene. In the absence of such clues, exome sequencing is a useful technique for finding the disease gene. Greater understanding of the mechanisms of CMS have been obtained from structural and electrophysiological studies of the endplate, and from biochemical studies. Present therapies for the CMS include cholinergic agonists, long-lived open-channel blockers of the acetylcholine receptor ion channel, and adrenergic agonists. Although most CMS are treatable, caution should be exercised as some drugs that are beneficial in one syndrome can be detrimental in another.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors reports a conflict of interest.
Figures



References
-
- Wood SJ, Slater CP. Safety factor at the neuromuscular junction. Prog Neurobiol. 2001;64:393–429. - PubMed
-
- Abicht A, Dusl M, Guergueltcheva V, et al. Congenital myasthenic syndromes: achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: a study of 680 patients. Hum Mutat. 2014;33:1474–1484. - PubMed
-
- Shinawi M, Cheung SW. The array CGH and its clinical applications. Drug Discov Today. 2008;13:760–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

